Curated OER
2001 U.S. National Chemistry Olympiad - Local Section Exam
Sixty multiple-choice chemistry questions make up this comprehensive exam used for the 2001 US National Chemistry Olympiad. Every topic that you would expect to approach in a general chemistry class is queried on. You could easily use...
Radford University
Is Fall Normal?
Fine the normality of fall measurements. Pairs collect measurements of fall leaves and one other fall object. Using the measurements, the groups determine the descriptive statistics for the object and using the Empirical Rule, figure out...
Beyond Benign
Can You Hear Me Now? Cell Phone Accounts
How sustainable are cell phones? Throughout the unit, learners explore the issues around cell phones concerning sustainability. Class members take a graphical look at the number of cell phones across the world using a box-and-whisker...
CK-12 Foundation
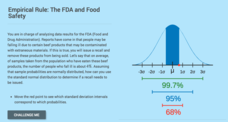
Computing Probabilities for the Standard Normal Distribution: The FDA and Food Safety
To recall or not to recall, that is the question. Using provided data, pupils calculate the percent of people that may fall ill on average. The scholars determine the standard deviation based upon the mean and the empirical rule, then...
Curated OER
Chapter 2 and 3 Practice Worksheet - Formulas and Compounds
In this formulas and equations activity, chemistry apprentices write the chemical formulas given the names. They calculate percent composition, balance equations, and calculate the mass of a substance present in a fertilizer. This is a...
Curated OER
Empirical Formula
Plenty of time was invested in creating this detailed presentation. The first slide alone includes several steps that support your explanation of percent composition, a precursor to understanding how to write empirical and molecular...
Curated OER
Oxidation Numbers and Ionic Compounds
For this oxidation numbers and ionic compounds worksheet, students write the formula for compounds given ions, they write the ions given formulas, they list oxidation numbers for elements, they write formulas for compounds and they name...
Curated OER
WS 5.5 Percent Composition
In this percent composition worksheet, students find the percentages of atoms in compounds and they determine empirical formulas given the percent of each atom in a compound.
Curated OER
Chemistry Practice
In this chemistry overview worksheet, students calculated the volume of different gases and liquids given in a word problem. Students had to calculate the molecular mass and write the empirical formula and the molecular formula.
Curated OER
Empirical Formula: From Percentage to Formula
Starting with definitions of empirical and molecular formulas, this slide show guides pupils through the ways to calculate and produce chemical formulas as percentages and molar ratios. A number of examples are given for viewers to try...
Curated OER
Empirical and Molecular Formulas
In this compounds worksheet, students write the empirical formulas or the molecular formula for the given chemical compounds. This worksheet has 20 problems to solve.
Curated OER
Percentage Composition and Empirical & Molecular Formula
In this compounds worksheet, students determine the empirical and molecular formulas for given compounds. This worksheet has 10 problems to solve.
Curated OER
Percentage Composition
In this chemical compounds worksheet, students determine the empirical formula or the molecular formula for the compounds given. Students calculate percent composition. This worksheet has 10 problems to solve.
Curated OER
Formulas from Masses
In this mass worksheet, learners determine the percentage composition of given compounds. Students write the empirical formula from the percentage composition. This worksheet has 5 problems to solve.
Curated OER
WS 4.8-Review
In this chemistry review worksheet, students solve a variety of problems including balancing equations, solving for unknowns using dimensional analysis, finding percent yields, limiting reactants and empirical formulas.
Curated OER
WS 4.5 Percent Composition and Empirical Formula
In this compounds instructional activity, students determine the empirical formulas and percent compositions of compounds given chemical formulas, mass of elements in compounds and percentages of elements in compounds.
Curated OER
Combustion Analysis Worksheet
In this combustion activity, students are given directions as to how to analyze a combustion reaction and they then solve five problems using this process.
Curated OER
Empirical and Molecular Formula Sheet
In this formulas worksheet, students solve eight problems and are required to write the empirical and molecular formulas given percentage of elements or quantities in mass.
Curated OER
Stoichiometry
In this stoichiometry activity, students review definitions and equations associated with molarity, density, atomic mass, molarity, and dilutions. This activity has 18 word problems.
Curated OER
Empirical and Molecular Formula
For this formulas worksheet, students review how to write compounds in their empirical formula and molecular formula. Then students complete 4 problems.
Curated OER
Chemical Equations and Stoichiometry
In this chemical equations worksheet, students calculate how many grams are used to in the equation written as well as the grams formed in the chemical equation. Students identify the limiting reagent in some of the chemical equations....
Curated OER
High School Chemistry Questions
In this chemistry worksheet, learners determine the number of water molecules is a given volume of water. Students determine the empirical formula for given compounds from the percentage mass of elements. This worksheet has 20 problems...
Curated OER
Chemistry 151 - Final Review
In this chemistry review worksheet, students give atomic symbols for given atoms, calculate moles, determine empirical formulas, and balance chemical equations. This worksheet has 1 drawing, 9 fill in the blank, and 12 word problems.
Curated OER
Chemical Equations
In this chemical equations activity, students determine the empirical formulas for chemical compounds and balance chemical equations. This activity has 1 fill in the blank question and 4 problems to solve.