Kenan Fellows
Sustainability: Learning for a Lifetime – The Importance of Water
Water is essential for life—and understanding the importance of clean drinking water is essential in understanding sustainability! Show your environmental science class the basics of water testing and treatment through a week-long...
Nuffield Foundation
Observing Water Moving Through Plants
We know plants assist in the water cycle, but how do plants get water from the ground into the air? Through a series of demonstrations or labs, scholars observe the movement of water through plants. They microscopically view the cells...
Science Matters
Oh Heron
Two teams—the environmentalists and herons—play four rounds of the game, Oh Heron. Using hand symbols to represent food, shelter, and water, players locate their match to produce more herons while those unmatched decompose.
DiscoverE
Clean It Up
Water, water, everywhere, but not a drop to drink—until we clean it, of course! Scholars design a filtration device that removes pollutants from water. The goal is to have the water come out as clean as possible from the device. How...
DiscoverE
Hold Your Water
Let's hope there are no leaks. Pupils work together in groups to build a device that will keep as much water as possible in a cup. After being dropped from a height of seven feet! Time to haul out the ladder.
Royal Society of Chemistry
pH 4: Activity
Sometimes playing games in class isn't a bad thing. Science sleuths evaluate and calculate pH and pOH with an online resource. They then manipulate concentration information and relate it during a series of puzzles.
Royal Society of Chemistry
pH and pOH
Feeling a little neutral about your pH and pOH teaching strategy? Perk it up with engaging puzzles! Young scientists relate ion concentration to pH and pOH, as well as the dissociation constant for water. The resource is available as an...
Serendip
Out Spot, Darn Spot
Encourage your classes to be laundry helpers! Learners explore the chemistry of stain removal with a lab investigation. By identifying the components of the stain, they identify the most effective solute for its removal.
DiscoverE
Pipe Maze
Here's a lesson that is simply a-MAZE-ing! Introduce science scholars to pipeline systems through a hands-on project. Partnered pupils participate in the design, construct, and test a PVC pipeline maze. Reusable materials and clear...
DiscoverE
Action Figure Diver
Will your next buoyancy lab rise to the occasion? Make a splash with action figure divers! Teams of young physicists explore the relationship between mass and buoyancy by adding weights or balloons to achieve a diver that neither sinks...
Colorado State University
Why Are Clouds White?
Is it possible to change the color of clouds? A three-part activity explores the scattering of light by the water droplets that make up clouds. After observing a demonstration, curious scholars conduct their own investigations of the...
Royal Society of Chemistry
A Reversible Reaction of Hydrated Copper (II) Sulfate
How can removing water change the color of a substance? Lab partners remove the water of crystallization from hydrated copper (II) sulfate, record their observations, then rehydrate the solid. The resource is printable and contains ideas...
Royal Society of Chemistry
Common Compounds
Can your young chemists identify the most commonly used chemicals in the lab? Introduce the class to the go-to substances in most middle and high school chemistry experiments with an interactive. The resource offers timely feedback as...
Royal Society of Chemistry
A Microscale Acid-Base Titration
Watch as acids and bases put smiles on their faces. Young chemists learn the concept of acid-base titration firsthand in a microscale experiment. Working groups collaborate, titrate, then use their data to determine the concentration of...
Royal Society of Chemistry
Investigating Temperature Changes on Evaporating Liquids—Microscale Chemistry
Is there more to evaporation than just less liquid? Show young scientists the energy transformation that occurs during a phase change through a series of simple experiments. Lab partners place drops of water, ethanol, and ethoxyethane on...
National Library of Medicine
Your Environment, Your Health: The Great Debate—Bottled Water vs. Tap Water in Our School
Should bottled water be sold in schools, or should they only provide tap water? The summative unit in the six-part series encourages scholars debating this topic. The lessons teach how to build an argument, how to gain background...
Concord Consortium
Chain Reaction Between Hydrogen and Oxygen
Looking for a simple way to teach conservation of energy in chemical reactions? Pupils can observe energy changes as water forms during a chain reaction between oxygen and hydrogen using an interactive. The resource instructs users to...
Concord Consortium
Dissolving
What happens to substances when they dissolve in water? Young scientists investigate the dissolving process with a colorful interactive. The resource illustrates changes in potential energy as solute particles interact with water...
Concord Consortium
Mixing Liquids
Sometimes being mixed up is a good thing! Young chemists explore the basics of making solutions with a realistic interactive. Pupils observe the differences in volume using quantities of water and ethanol, as well as the effects of...
Concord Consortium
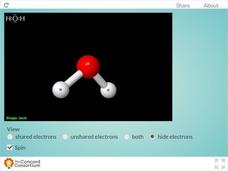
Unshared Electrons and the "Bent" Shape
Why is water always so bent out of shape? Scholars investigate the molecular geometry of the water molecule using a 3-D resource. The interactive features options such as rotation and the ability to view electron pairs.
Concord Consortium
Oil and Water
If you don't get along with someone, it's said that the two of you are like oil and water. Why is this? Explore the phenomenon and explain the phrase in one resource! Science superstars first observe samples of oil and water together....
Concord Consortium
Molecular Sorting
Can scientists sort molecules based on their interaction with oil and water? The simulation demonstrates how this is possible. Pupils decide when to insert a molecule and observe how they sort themselves based on polarity.
Concord Consortium
Micelles
Micelles consist of an aggregate of molecules in a colloidal solution. The simulation presents two different ways the molecules assemble into micelles based on the polarity of the solution in which they are placed. Scholars can set the...
Concord Consortium
Polar and Non-Polar Interface
Why is there so much frozen water at Earth's poles? Because water is a polar molecule! Young scientists observe polar molecules moving in a mixture of oil and water. They see the changes in potential energy in the hydrophilic and...