Curated OER
Typical Conceptual Questions for Physics I - Light and Quantum
This is a stellar overview of everything light and quantum! There are 30 multiple choice questions, none of them requiring any mathematical computation. There are a few diagrams to analyze: light rays striking reflective and refractive...
Curated OER
Using the Spectrophotometer to Analyze a Mixture
General chemistry classes practice spectroscopy. They grasp the relationships between wavelength, absorbance, and solution concentration. Additionally, they gain valuable practice using laboratory equipment such as burets and pipettes....
Curated OER
2009 U.S. National Chemistry Olympiad National Exam - Part I
The 2009 version of the first part of a national chemistry competition is posted for your use with olympiad hopefuls. Test takers deal with 60 multiple choice questions covering an entire year of chemistry curriculum. Use this to...
Curated OER
How Big Is That Star?
Aspiring astronomers study stars. They compare stars and explain the relationship between radius, mass, and diameter. By creating a star simulation, they discover how a binary star system's orbit can cause changes in the observed...
Curated OER
Supernova Chemistry
Using spectroscopes, high school astronomy, physics, or chemistry learners observe emission spectra from several different sources. This stellar NASA-produced lesson plan provides terrific teacher's notes and a student handout. Make sure...
Curated OER
X-ray Spectroscopy and the Chemistry of Supernova Remnants
This link takes you to a comprehensive unit that delves into emission spectra and supernovas. There are four parts: How and where elements are created, electromagnetic radiation, spectroscopy, and the newest technology for studying our...
Curated OER
Atomic Absorption Determination of Zinc and Copper in a Multivitamin
Advanced lab apprentices prepare zinc and copper solutions to which they will compare the same minerals from a multivitamin. Using absorption spectroscopy, they analyze the contents of the multivitamin for concentration. This lab can be...
Curated OER
Energy
Wow! Colorful and simple, these 160 slides introduce the various forms of energy, along with a relevant image. Some of the images are animations, which help beginning physical scientists to visualize the flow of electrons or energy! This...
Curated OER
Typical Numeric Questions for Physics I - Atomic Spectra
Seven practice problems are presented to physics pros in this assignment. Given the wavelengths, they perform computations for emission spectra. This brief activity makes an appropriate pop quiz.
Curated OER
Organic Chemistry Problem Set Exam 1
Though there are technically only 13 questions on this exam, they take up six pages and make a thorough assessment of organic chemistry principles. There are plenty of diagrams to label or complete. Emission spectra are displayed for...
Curated OER
Typical Numeric Questions for Physics I - Photoelectric Effect
As the title implies, here is a collection of typical photoelectric effect problems that physics learners need to be able to solve. They determine the amount of energy of a photon, the photons produced per second, the frequency required...
Curated OER
Emission Spectra
These attractive slides explain the basics of the electromagnetic spectrum and then display the emission spectra for a number of elements. argon, helium, hydrogen, xenon, neon and krypton are shown here and two graphs showing log scale...
University of Colorado
University of Colorado: Physics 2000: Spectral Lines
Several pages from an excellent site which describe the science of spectroscopy. The unique atomic emission (and absorption) line spectrum of elements are illustrated and explained. Includes a Java applet depicting the quantum energy...
University of Colorado
University of Colorado: Physics 2000: Quantum Atom
Several pages with an interesting discussion of the visible light spectrum and atomic absorption and emission line spectrum. Features excellent graphics, thorough and understandable discussion, and many interactive Java applets.
Georgia State University
Georgia State University: Hyper Physics: Hydrogen Energies and Spectrum
This site from Georgia State University gives information on the transitions of electrons between energy levels. The energy levels for electrons in the hydrogen atom are discussed. The Rydberg equation is stated and electron transitions...
University of Colorado
University of Colorado: Physics 2000: Balmer's Formula
A short description of a physics lab involving the determination of the wavelength of the four spectral lines in the hydrogen emission spectrum. Perhaps the most important part of the page is the picture of the spectrometer.
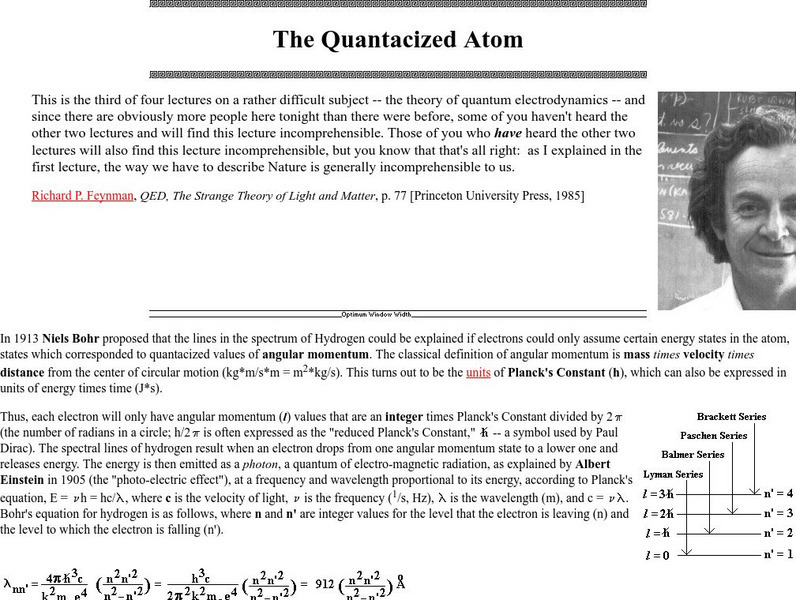
Friesian School
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
McGraw Hill
Mc Graw Hill Companies: Origins of the Quantum Theory
An indexing page for Chapter 27 (Origins of the Quantum Theory) of the companion web site for McGraw Hill's Contemporary College Physics textbook. Includes several worthy pages with chapter notes, simple definitions, online computer...
Other
Brockport High School: Energy Levels of Hydrogen Atom
From the Brockport High School Physics Labs web pages. Includes an excellent graphic depicting the energy levels of a hydrogen atoms and portraying the electron level transitions for the Lyman, Balmer, and Paschen series. Includes both...
University of St. Andrews (UK)
University of St. Andrews: Johann Jakob Balmer
Describes the life and scientific contributions of Johann Jakob Balmer. Discussion focuses on Balmer's contributions to the line spectra of hydrogen.