Curated OER
Protons, Neutrons and Electrons
For this chemistry worksheet, students use the periodic table to complete the chart shown, filling in all of the missing information in the blank spaces. They identify and describe various elements found in the periodic table.
Curated OER
Liquids and Solids
Students describe the properties of solids and liquids, and explain how a semiconductor works. In this atom lesson students demonstrate the bonding properties of carbon and silicon.
Curated OER
Chapter 22 - 25 Review
For this chemistry review worksheet, students explore their knowledge of solutions, acid and bases, nuclear reactions, and radioactive decay as they answer 33 matching, fill in the blank, and short answer questions.
Curated OER
Oxyanions
In this oxyanions worksheet, students identify the common polyatomic ions. They write formulas and name polyatomic ions based on IUPAC rules.
Curated OER
Periodic Table Basics 2
In this periodic table instructional activity, students create cards for a annotated periodic table then use it to answer 16 short answer and fill in the blank questions.
Curated OER
Simple Chemical Equations
Eighth graders interpret the chemical symbols and shorthand used for molecules and equations. Through guided practice, 8th graders work to decipher and interpret the symbols for chemical equations. An opportunity for independent...
Curated OER
Naming Inorganic Compounds
In this inorganic compounds activity, students complete 2 charts by writing the names or chemical formulas of given ionic and covalent compounds.
Curated OER
Ammonium Nitrate - Heat of Solution
Students quantify the relationship between temperature, energy and heat
and define an endothermic reaction. They measure the energy change caused by dissolving one mole of ammonium nitrate in water.
Curated OER
Using several learning modalities to teach about the Periodic Table.
Students identify how to relate the position of an element in the periodic table to its atomic number and atomic mass. They identify how to use the periodic table to identify metals, semimetals, nonmetals, and halogens, and also,...
Curated OER
Periodic Table
Students relate to the idea of periodicity & give example of other periodic events and identify and use the periodic table as a useful resource. They also identify the relationship of elements to certain groups & periods &...
Virginia Department of Education
Isotopes
Lead your class through the amazing world of isotopes as they investigate the various properties they contain and further understand their respective location on the periodic table. They explore half-lives and radioactivity as each...
Virginia Department of Education
Radioactive Decay and Half-Life
Explain the importance of radioactive half-life as your high school biologists demonstrate the concept by performing a series of steps designed to simulate radioactive decay. Pupils use pennies to perform an experiment and gather data....
Science Geek
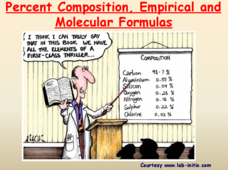
Percent Composition, Empirical and Molecular Formulas
Help your pupils understand when empirical becomes molecular. The lesson presentation demonstrates the connection between empirical formulas and molecular formulas. Then, given percent composition, the lesson demonstrates the steps to...
Science Geek
Valence Electrons
There is a lot of negativity when studying electrons, but this presentation makes the experience more positive by beginning with the definition of a valence electron and breaking down the number of valence electrons by groups on the...
Virginia Department of Education
Formulas and Percent Compositions of Ionic Compounds
Try not to blind anyone with science by following the safety rules. The lesson encourages scholars to form an ionic compound from magnesium and chlorine. Then they determine the empirical formula and determine the mole ratio and percent...
Curated OER
Unit 2 ~ Atomic Structure
As an atomic structure reference and review tool, this handout fits the bill. The first page provides definitions and tables of orbitals, electrons, and energy levels. The second page is an opportunity to practice determining numbers of...
Curated OER
Relative Mass Formula, Atomic Formula, and Empirical Formula
After giving the definitions of the different compound terms and formulas, equations are provided to teach your chemists to calculate different values. Relative formula mass, atomic mass, and empirical mass are shown and explained with...
Curated OER
Beat the Greeks
Students conduct research of the history of atomic theory. Information is presented from Democritus and Aristotle to the early Renaissance using the Internet and video.The integration of technology allows for a vast amount of research...
Curated OER
Date a Rock!
Young scholars figure the number of half-lives since the sample solidified, and therefore the "age" of the sample rocks.
Science Geek
Balancing Chemical Equations
Balancing equations means conserving mass; use the presentation to help learners practice this concept. The resource includes 56 equations for your class to practice balancing. Scholars focus on one reaction type at a time. Groups of...
Science-Class.net
Rock Candy Crystals
Candy is one of my favorite words, and it's an even better word when it relates to science. Yes, candy science can happen when you grow rock candy crystals with your class. The entire process for growing these edible wonders of nature is...
Curated OER
Activity #4 What Do Equations Mean?
Students practice with writing and balancing simple chemcial equations. they comprehend that chemical equations are a method of using a set of univeral symbols to represent what happens experimentally in chemical reactions. Pupils...
Curated OER
The Energy Debate - Stoichiometry
Students determine crude oil can be separated into useful fractions by a process of fractional distillation. They write a balanced equation for the reaction between a hydrocarbon and oxygen.
Curated OER
Radioactive Decay and Half-Life
Students describe how the mass of a radioactive isotope changes with time and the factors that affect the rate of radioactive decay. They write nuclear decay equations to represent natural transmutation. This activity is accomplished...