Kenan Fellows
What Element Would You Be?
Primo Levi wrote a collection of short stories comparing his life from Italy to Auschwitz to elements in the periodic table. Pupils read an excerpt from his book and research the characteristics of various elements. Then, they make a...
Curated OER
Biologically Important Molecules
In this biologically important molecules learning exercise, students fill in the blank with information about carbohydrates, lipids, and proteins. Students also make notes about nucleic acids.
Curated OER
An Introduction To Material Sciences
Students investigate the concept of molecular structure. They describe the behavior of simulated molecules and identify any dislocations in crystalline structures. They also define various related vocabulary words and use them in...
Curated OER
How is the Strength of an Acid Determined?
Young scholars study acids and how they can be measured. In this acid lesson students distinguish the properties that create strong and weak electrolytes.
Curated OER
Chemical Formulas Unit-Day 4
The purpose of this lesson plan involves reviewing nomenclature and molecular
formulas as well as introducing naming molecular compounds and identifying acids. The students investigate and understand how conservation of energy
and matter...
Mr. E. Science
Acids, Bases and Solutions
If you are not part of the solution, then you are part of the precipitate. The presentation covers solutions, suspensions, solubility, dissociation, and acid/base reactions. This is the 19th lesson in a series of 26.
Curated OER
BioFuels: The Chemistry and Economics of Alternative Fuels
Junior chemists manufacture biodiesel in the lab. In this exercise, they check the purity of the biodiesel using thin layer chromatography. They also calculate its density and heat of combustion. They are sure to rise to the challenge...
Curated OER
Writing Chemical Formulas
Students study how to write chemical fomulas by reviewing the combinations atoms form into compounds. They write a procedure to test various substances and name the compounds and write formulas. As they construct models for formulas and...
Curated OER
Phases of Matter
For this matter worksheet, students calculate volume and partial pressure for gases, compare liquid and vapor phases, and review the characteristics properties of gases. This worksheet has 12 multiple choice and 3 problems to solve.
Curated OER
Tie Dye
Students practice writing research proposals to test the color fastness of a dye once it has been exposed to a t-shirt. Each proposal needs details of experimental design, length of treatment, and means of cleaning the shirt. All...
Curated OER
VSEPR And Polarity
For this VSEPR theory worksheet, students evaluate the electron-pair geometry of organic and inorganic molecules. They construct Lewis structures and resonance structures for 17 compounds and complete 3 short answer questions.
Curated OER
Chemical Formulas
Students assess how to go from writing ionic chemical formulas to nomenclature and naming chemical compounds. They brainstorm former studies and share their thoughts in small groups. A list of everyday products (shampoos, toothpaste,...
Curated OER
Water - the (Nearly) Universal Solvent
In this water worksheet, high schoolers explore the reasons why water is considered a universal solvent. Students compare different ways to change the dissolving rate of a solute. This worksheet has 11 fill in the blank and 8 matching...
Curated OER
Characteristics of Crystals
In this crystals worksheet, students complete a graphic organizer by filling in the characteristics of the different crystal types including melting/boiling point and electrical conductivity.
Curated OER
Physical and Chemical Properties of Water
High schoolers experiment with water as a component of suspensions, solutions, and heat conduction contributing to the use of food and the health and wellness of human beings.
Curated OER
Doing Lewis Dot Diagrams
Students observe the periodic table and draw the Lewis Dot Diagram. In this investigative lesson students construct information on several elements including the Lewis Dot Formation and take a quiz on the information they learned.
Curated OER
Water - the (Nearly) Universal Solvent
In this solvent worksheet, students explore why water is considered a universal solvent. Students explore what can change dissolving rates. This worksheet has 8 matching, 3 short answer, 11 fill in the blank, and 4 problems to solve.
American Chemical Society
Middle School Chemistry: Energy Levels, Electrons, and Covalent Bonding
Discover how covalent molecular bonding affects the energy levels of electrons.
Concord Consortium
Concord Consortium: Stem Resources: Chemical Bonds
By working through this web-based activity, students differentiate between ionic, non-polar covalent, and polar covalent bonds. Specifically, distinctions are made between bonding types based on orbital shapes and electronegativity...
Wisc-Online
Wisc Online: Lewis Dot Structures of Covalent Compounds
Short slide show provides basic information about drawing Lewis dot structures for covalent compounds. Starts with anatomy of the atom, and then shows the relationship between atomic particles and the Periodic Table of Elements. Offers...
PBS
Pbs Learning Media: Molecular Shapes
In this interactive activity from ChemThink, students will learn about covalent molecules and how the VSEPR theory predicts the shapes of covalently-bonded molecules.
Michael Blaber, PhD
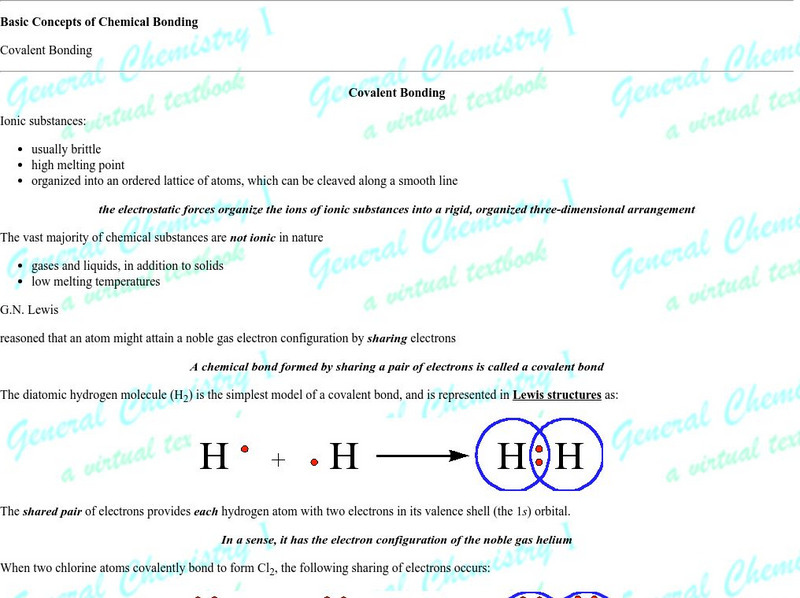
Florida State University: Basic Concepts of Covalent Bonding: Covalent Bonding
Good introduction and graphics make this a solid page for understanding the orbital role in bonding and molecular geometry. The author is a professor at Florida State University.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molecular Structure [Pdf]
The Molecular Structure chapter from "An Introduction to Chemistry" textbook discusses the formation of covalent bonds, drawing Lewis dot structures, and the resonance and molecular geometry of molecules. Many pictures and examples are...
CK-12 Foundation
Ck 12: Physical Science: Molecular Compounds
[Free Registration/Login may be required to access all resource tools.] Definition of covalent compound, how they are named and how they differ from ionic compounds.