New York University
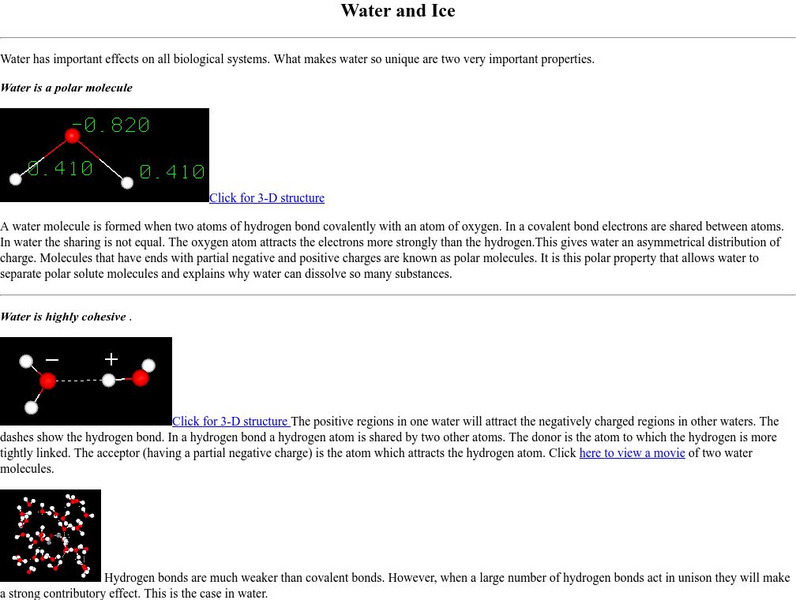
New York University: About Water and Ice
Page uses movies and 3D images to explain how properties of water relate to polarity and hydrogen bonding.
TED Talks
Ted: Ted Ed: Why Does Ice Float in Water?
Ever wonder why solid ice floats in liquid water? In this video lecture you will explore the special properties of water. Learn "the science behind how hydrogen bonds keep the ice in your glass (and the polar ice caps) afloat". At the...
Khan Academy
Khan Academy: Test Prep: Mcat: Chemical Processes: Covalent Bonds: Intramolecular and Intermolecular Forces
Explains what intramolecular and intermolecular forces are and the different types for each. Includes lots of examples.
University of Southern California
Atomic Bonds
This slide show on atomic bonds contains several slides on electron affinity. Other topics include covalent, Sigma and Pi bonds, and atomic bonding in solids.
Chem4kids
Chem4 Kids: Hydrogen
Here you can find out about hydrogen, the first element in the periodic table. Content includes shell information, where to find it in nature, and why it is helpful to us.
CK-12 Foundation
Ck 12: Water Properties
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will describe the structure and polarity of a water molecule. They will also describe the hydrogen bonding that occurs in...
TeachEngineering
Teach Engineering: Density & Miscibility
After students conduct the two associated activities, Density Column Lab - Parts 1 and 2, present this lesson to provide them with an understanding of why the density column's oil, water and syrup layers do not mix and how the concepts...
Frostburg State University
General Chemistry: Why Oh, Nh, and Fh Bonds Are Polar
Frostburg State University provides a brief explanation to the question of why small hydrogen molecules, such as OH, NH, and FH, are very polar.
Other
What Is Life: Aqueous Solutions
Discussion of water as a solvent. Includes material on hydrogen bonds, hydration, hydrophobic effect, acids, basses, and pH.
American Chemical Society
Middle School Chemistry: Energy Levels, Electrons, and Covalent Bonding
Discover how covalent molecular bonding affects the energy levels of electrons.
John Wiley & Sons
Concepts in Biochemistry: Concept Reviews: Water, P H, and Non Covalent Bonding
A detailed review of water and its structure. Included are diagrams, animated graphics, and review quizzes. For the advanced high school student.
Curated OER
Science Kids: Science Images: Hydrogen Bonds
A computer generated image showing a 3D model of hydrogen bonds in water.
CK-12 Foundation
Ck 12: Chemistry: Physical Properties of Water
[Free Registration/Login may be required to access all resource tools.] Covers physical properties of water, hydrogen bonding, surface tension, and vapor pressure.
CK-12 Foundation
Ck 12: Chemistry: Structure of Water
[Free Registration/Login may be required to access all resource tools.] Covers structure of water molecule, hydrogen bonding on water, polarity of water molecule, and bond angles of water molecule.
CK-12 Foundation
Ck 12: Water and Life
[Free Registration/Login may be required to access all resource tools.] The chemical and physical properties of water are examined in this lesson, including the role of hydrogen bonds in determining water's properties. The importance of...
CK-12 Foundation
Ck 12: Chemistry: Structure of Ice
[Free Registration/Login may be required to access all resource tools.] Covers structure of ice and hydrogen bonding in ice.
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 2
This lesson will present the basic properties and characteristics of chemical bonds. It is 2 of 4 in the series titled "Characteristics of Chemical Bonds."
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 4
This lesson will present the basic properties and characteristics of chemical bonds. It is 4 of 4 in the series titled "Characteristics of Chemical Bonds."
Sophia Learning
Sophia: Molecular Structure of Water: Lesson 2
This lesson will introduce the molecular structure of water, including the Hydrogen atoms, Oxygen atoms, and types of bonds. It is 2 of 4 in the series titled "Molecular Structure of Water."
Sophia Learning
Sophia: Molecular Structure of Water: Lesson 3
This lesson will introduce the molecular structure of water, including the Hydrogen atoms, Oxygen atoms, and types of bonds. It is 3 of 4 in the series titled "Molecular Structure of Water."
University of Arizona
University of Arizona: Biochemistry
Problem sets, tutorials, and activities related to biochemistry.
Sophia Learning
Sophia: Molecular Structure of Water
A brief introduction to the molecular structure of a water molecule, and the chemical bonding which gives it its unique properties.
University of Arizona
Ua: Chemistry Tutorial
This general tutorial begins with an explanation of the polarity of the water molecule and the effects this polarity has on the properties of water. Goes on to introduce organic molecules and has a thourough tutorial on the third page.
Khan Academy
Khan Academy: Biology: Water, Acids, and Bases: Cohesion and Adhesion of Water
This article defines and discusses cohesion and adhesion. Understand these two topics and their importance in hydrogen bonding.