Virginia Department of Education
The Rate of a Chemical Reaction
If your pupils think a catalyst is a list of their cats, then this might be the lesson for you! Young chemists study the effect of temperature, catalysts, concentration, and particle size on reaction rates during four different...
Virginia Department of Education
Soap, Slime, and Creative Chromatography
Do you think chromatography paper suffers from separation anxiety? Young chemists make soap, slime, silly putty, and experiment with chromatography in this instructional activity. The material includes clear instructions for each...
Curated OER
Using several learning modalities to teach about the Periodic Table.
Students identify how to relate the position of an element in the periodic table to its atomic number and atomic mass. They identify how to use the periodic table to identify metals, semimetals, nonmetals, and halogens, and also,...
Curated OER
Pauli's Magical Water
Students predict the shape of molecules using VSEPR theory. In this chemistry lesson, students differentiate a polar and nonpolar molecule. They discuss why water's polarity is very important.
Curated OER
Comparing the Degree of Unsaturation of Margarine with that of Butter
Is butter better? In terms of saturation, young chemists find out! Using titration methods, they will compare the degree of unsaturation of butter with that of margarine. Knowing the unsaturation, they can make conclusions about the...
Virginia Department of Education
Heat Transfer and Heat Capacity
It's time to increase the heat! Young chemists demonstrate heat transfer and heat capacity in an activity-packed lab, showing the transitions between solid, liquid, and gaseous phases of materials. Individuals plot data as the changes...
Curated OER
Natural Indicators: How Do They Work?
Students describe characteristics and common uses of acids and bases. They describe the role of natural indicators in the chemistry of acids and bases after testing and making observations on a variety of plant extracts. Students observe...
Curated OER
Phases of Matter
For this matter worksheet, students calculate volume and partial pressure for gases, compare liquid and vapor phases, and review the characteristics properties of gases. This worksheet has 12 multiple choice and 3 problems to solve.
Curated OER
Infrared Spectroscopy in Forensic Chip Analysis
Students analyze spectrums as related to forensics. In this chemistry lesson, students define spectroscopy and discuss its use in chemistry. They discuss how the Forensic Paint Chip is used to help solve crimes.
Other
Monterey Peninsula College: Molecular Orbital Theory
Very deep presentation of molecular bonding and orbital theory. Nice graphics and text make it a good presentation of some high end material.
State University of New York
State University of New York: Molecular Orbital Theory
This exercise displays the molecular orbitals for a few small molecules and examines how to classify each.
Frostburg State University
General Chemistry Online: Chemical Bonds Faq
Find answers to common questions about chemical bonds. Recognize molecular orbital theory and know how to draw a Lewis structure.
PBS
Pbs Learning Media: Molecular Shapes
In this interactive activity from ChemThink, students will learn about covalent molecules and how the VSEPR theory predicts the shapes of covalently-bonded molecules.
Simon Fraser University
Chem1 Virtual Textbook: Molecular Geometry
An advanced explanation of the valence shell electron pair repulsion (VSEPR) theory describes specific molecular models involving digonal, trigonal, tetrahedral, and octahedral coordination, as well as central atoms with five bonds....
CK-12 Foundation
Ck 12: Molecular Geometry
[Free Registration/Login may be required to access all resource tools.] In this interactive learning module, students will learn a technique to predict molecular geometry based on a molecule's Lewis electron dot structure.
Michael Blaber, PhD
Florida State University: Molecular Geometry and Bonding Theories
This article from the Florida State University contains information on multiple bonds and orbitals. Great charts and pictures are shown to help the educational process. Definitely a great site to check out on the subject.
CK-12 Foundation
Ck 12: Molecular Geometry
[Free Registration/Login may be required to access all resource tools.] The following online tutorial explains the basis of VSEPR theory. It helps students predict the shapes of molecules and polyatomic ions using VSEPR theory and it...
Towson University
Towson University: Shapes of Molecules
This chemistry class printout details the main points of molecular geometry and explains bond hybridization and bond angles.
Michael Blaber, PhD
Fsu: Molecular Geometry and Bonding Theories: Polarity of Molecules
Florida State University provides a discussion of polarity of binary and polyatomic molecules, with tables and graphics.
New York University
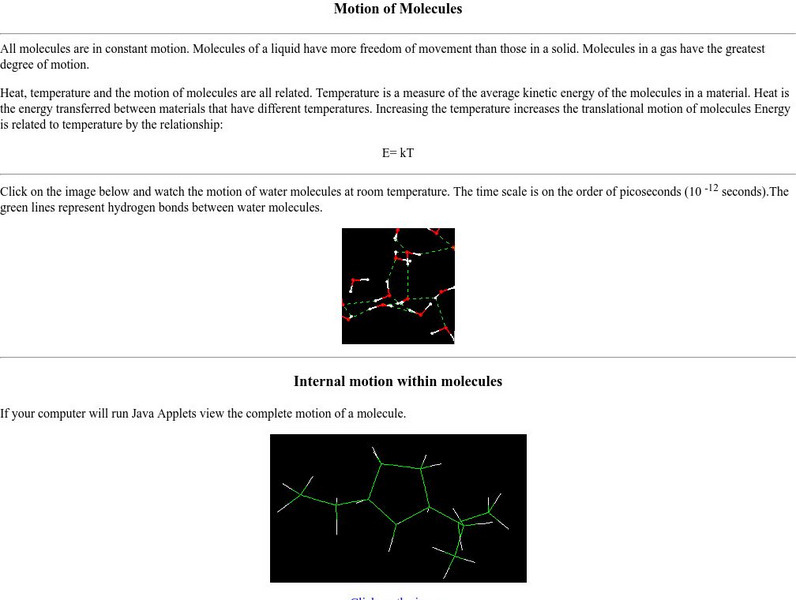
Nyu: Math Mol: Motion of Molecules
Examine the link between molecular motion and energy. Observe the movement of a molecule at room temperature. Learn about the different types of molecular motion.
Cosmo Learning
Cosmo Learning: Ap Chemistry With Chemguy
Need to review a topic for AP Chemistry? This site features a collection of video lectures covering the curriculum of AP Chemistry. The lectures are broken up by topics and vary in length. Lectures cover thermodynamics, bonding,...