Alabama Learning Exchange
Alex: Get in the Mix
Students will be able to classify a mixture as homogeneous or heterogeneous. Students will be able to create a solution. Students will be able to identify the solute. Students will be able to identify the solvent. Students will be able...
CK-12 Foundation
Ck 12: Osmosis
[Free Registration/Login may be required to access all resource tools.] Tutorial defines osmosis and the difference between osmosis and diffusion. Students will learn how to distinguish solute from solvent and solution and the hypotonic,...
CK-12 Foundation
Ck 12: Plix: Solute and Solvent
[Free Registration/Login Required] While paying attention to the charges, move the salt ions and water molecules around to simulate how salt ions are dissolved in water.
Carnegie Mellon University
Chem Collective: Brownian Motion
Particulate level simulations that show only solute particles are convenient, since they focus student attention on the molecules of most interest. However, such solute molecules move in a Brownian manner. This simulation helps students...
American Chemical Society
Middle School Chemistry: Lesson Plans: Does Temperature Affect Dissolving?
Students identify and control variables to design an experiment to see whether the temperature of a solvent affects the speed at which a solute dissolves.
TeachEngineering
Teach Engineering: Chromatography Lab
To increase students' awareness of possible invisible pollutants in drinking water sources, students perform an exciting lab requiring them to think about how solutions and mixtures exist even in unsuspecting places such as ink. They use...
Frostburg State University
Why Does the Solubility of Gases Usually Increase as Temperature Goes Down?
This chemistry course article, "Why does the solubility of gases usually increase as temperature goes down?," provides text and related links on the effects of temperature and pressure on the solubility of gases.
Concord Consortium
Concord Consortium: Molecular Workbench: Water and Polar Substances
Adjust amounts of ionic charges in this simulation to see how water molecules react to polar substances in solution.
University of Virginia
University of Virginia: More on Temperature and Solubility
Lab set up to compare the effect of temperature on the solubility of two solutes (NaCl and KNO3).
Frostburg State University
University of Frostburg: How Nonpolar Molecules Dissolve
This site from the University of Frostburg provides an explanation of the process by which nonpolar molecules dissolve in water.
Savvas Learning
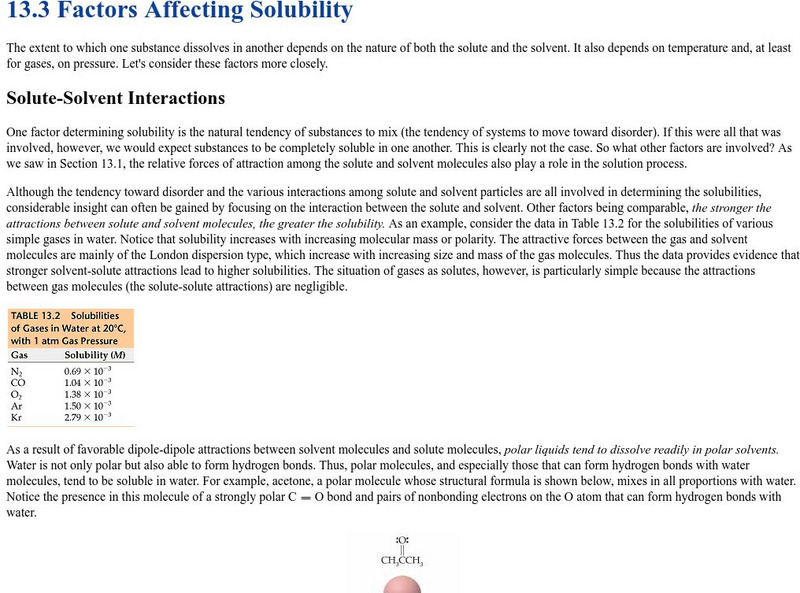
Pearson Education: Factors Affecting Solubility
Learn how the extent to which one substance dissolves in another depends on the nature of both the solute and the solvent.
Science Education Resource Center at Carleton College
Serc: Investigating Chromatography: Selecting Variables
For this lab, students will demonstrate observation skills as they design an experiment to separate colors of various water-based pens in order to learn about mixtures and solutions. Students will determine a variable to test and...
CK-12 Foundation
Ck 12: Chemistry: Dissolving Process
[Free Registration/Login may be required to access all resource tools.] Describes solution formation and interaction of solute with water.
Science Education Resource Center at Carleton College
Serc: Mn Step: Solutions: Solubility and Miscibility
For this activity, learners will test the solubility and miscibility of various substances in water, and explain the chemistry involved. They will also be given a solid and a liquid and asked to predict what will happen when each is...
Science Education Resource Center at Carleton College
Serc: Mn Step: Spotting Chromatography
A lab activity that introduces chromatography using markers, plus water and acetone as the solvents. Learners will take measurements to compare the mobile and stationary phases of the inks, and find the polarity of the inks and solvents....
Other
Aqueous
A chemical tutorial describes the term "aqueous" and features examples of how different substances dissolve.
Other
Science House: Ice Cream
Experiment shows students how to use the lowered freezing point of water to chill another mixture (ice cream) to the solid state. Teacher's notes provide background information.
CK-12 Foundation
Ck 12: Colloids and Suspensions
[Free Registration/Login may be required to access all resource tools.] In this lesson, students expand their study of mixtures to show that solids and gases can also act as solvents. Additionally, they take a look at situations in which...
American Chemical Society
Middle School Chemistry: Lesson Plans: Why Does Water Dissolve Salt?
Students use their own model of a salt crystal and water molecule to show how water dissolves salt. Then, they relate their observations to the structure of salt, water, and alcohol on the molecular level.
Georgia Department of Education
Ga Virtual Learning: Analysis of Organic Materials Analysis of Organic Materialsv
This comprehensive interactive tutorial continues to explore the forensic science field. Learn how organic materials of evidence are analyzed and what the various types of chromatography are, especially why they are so important to this...
Annenberg Foundation
Annenberg Learner: Virtual Particle Lab: Dissolving
Explore what happens when one substance dissolves into another. Run the simulations and see if you can predict the results.
Khan Academy
Khan Academy: Molarity vs. Molality
Learn how molarity and molality differ. The molality of a solution is equal to the moles of solute divided by the mass of solvent in kilograms, while the molarity of a solution is equal to the moles of solute divided by the volume of...
Estrella Mountain Community College
Online Biology Book: Chemistry Ii: Water and Organic Molecules
Online biology textbook discussing the chemical nature of water, and the importance of its molecular structure to life. Also discusses at length the organic molecules nucleic acids, proteins, lipids, and carbohydrates.
Other
Reverse Osmosis and Osmotic Pressure What They Are
This site gives a good explanation of osmosis and reverse osmosis. Also includes a scientific diagram of the process.
Other popular searches
- Solutions Solvent Solute
- Solute and Solvent
- Solute Solvent
- Solute and Solvent Lab
- Solution, Solvent, Solute
- Solution Solvent Solute
- Chemistry Solute and Solvent
- Solute Solvent Solution Lab
- Solute Solvent and Solution
- Solutions, Solvent, Solute
- Solvent and Solute Worksheet
- Solute Solvent Soluble