Chem Tutor
Chem tutor.com: Atomic Structure
This page provides a thorough and comprehensible discussion of the structure of the atom. Includes helpful diagrams and instructions for filling electron shells, reading the Periodic Table, and drawing Lewis Structures.
CK-12 Foundation
Ck 12: Electron Configuration and the Periodic Table
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will sse the Periodic Table to identify and explain the properties of chemical families, including alkali metals, alkaline...
Other
Chemistry Is Easy!: How Do You Write Electron Configurations?
With the goal of making chemistry understandable, this site guides the learner through electron configurations with layman's terms, colorful illustrations, and questions to check learning along the way. The "Purney Cheer" is also...
Georgia State University
Georgia State University: Hyper Physics: Hund's Rules
A complete overview of Hund's rules including exceptions.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Complete Electron Configurations Help
This section from the online textbook "An Introduction to Chemistry", provides some helpful hints to writing complete electron configurations. Three ways to predict the order of filling electron orbitals are given.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Multi Electron Atoms: Audio Book
This audio book, narrated by author Mark Bishop, describes how to write regular and abbreviated electron configurations, and how to draw orbital diagrams. Also find links to animations, tutorials, power points, and chapter maps about...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Complete Electron Configuration
Test your knowledge of common atoms and their corresponding electron configurations. In this interactive exercise, you will find out how much you really know about the orbitals of electrons.
Environmental Chemistry
Periodic Table of Elements: Gallium
A very detailed look at the element Gallium, a member of the Boron Group.
Environmental Chemistry
Periodic Table of Elements: Indium
A very detailed look at the element Indium, a member of the Boron Group.
Environmental Chemistry
Periodic Table of Elements: Thallium
A very detailed look at the element Thallium, a member of the Boron Group.
Purdue University
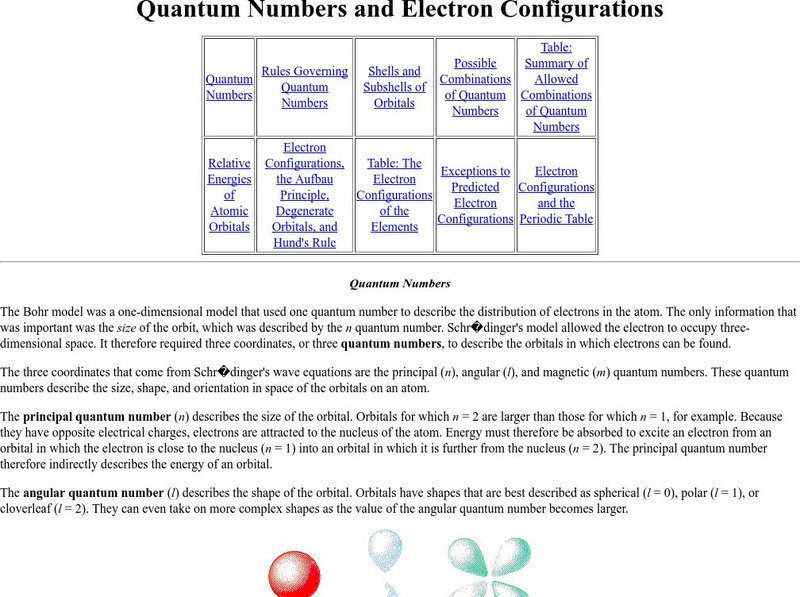
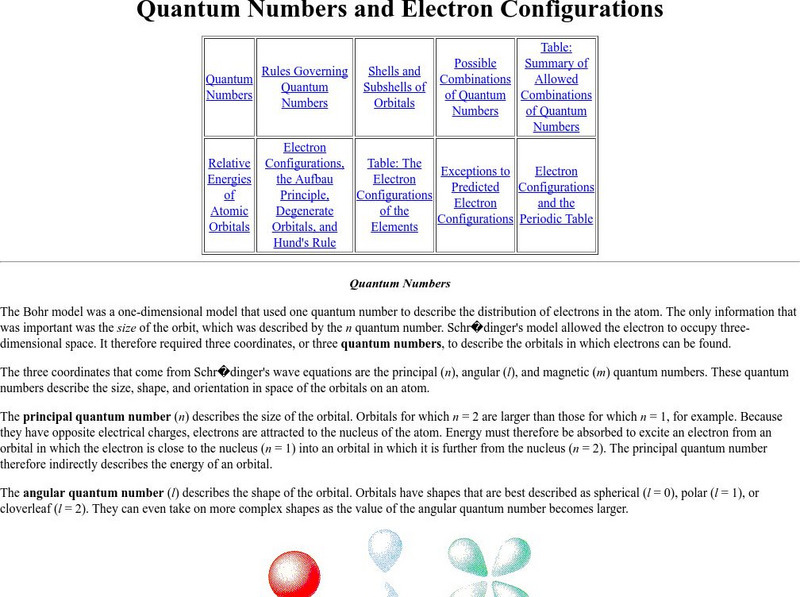
Purdue University: Quantum Numbers & Electron Configurations
This site from the Purdue University provides quantum numbers explained in detail and shown how they specify the atomic orbital of each electron and their energy levels. Electronic configurations are explained. Learning exercises with...
Purdue University
Purdue University: Orbital Shells
This site from the Purdue University provides an overview of atomic orbitals and how quantum numbers specify these orbitals. Includes a model that can be rotated and examined in 3D. Includes learning exercises and answers.
Purdue University
Purdue University: The Aufbau Principle
At this site from Purdue University, Electron Configurations, the Aufbau Principle, Degenerate Orbitals, and Hund's Rule are detailed. Includes learning exercises and answers.
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Exceptions to the Octet Rule
Lists the exceptions to the octet rule and provides a discussion and diagrams explaining each one. Includes clear diagrams illustrating this concept.
Michael Blaber, PhD
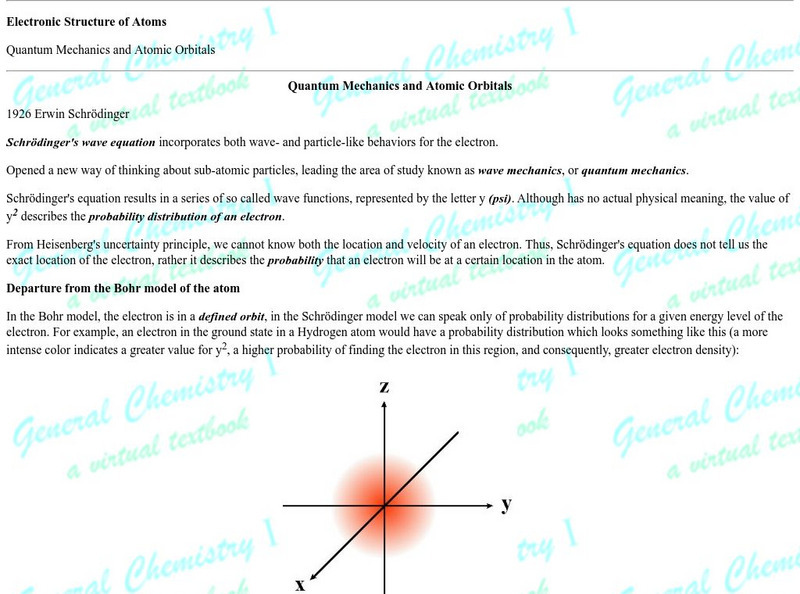
Fsu: Electronic Structure of Atoms: Quantum Mechanics & Atomic Orbitals
This article is provided by Florida State University. Schroedinger wave equation is explained and how quantum numbers are used to describe the energy level of any electron in an atom. The relationship of atomic orbitals to quantum...
Michigan State University
Michigan State University: Elementary Physics Ii: The Pauli Exclusion Principle
The Pauli Exclusion Principle requires that all electrons in an atom have unique energy levels.
Other
University of Delaware Physics Department: Lithography
University of Delaware Physics Department provides this indexing page for a slide show presentation.
Other
Univ. Of Delaware Physics: More Semiconductor Physics
The University of Delaware Physics Department provides this site from a site titled "Silicon, Circuits, and the Digital Revolution," here is a series of four pages which explain the details behind how semiconductors work. An introduction...
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
Other
Us: Valence Shell Electron Pair Repulsion (Vsepr)
An excellent tutorial that examines VSEPR and pairs of valence electrons. The valence shell electron pair repulsion concept is explored using animated models. Includes a VSEPR calculator. Use the toolbar on the left to navigate through...
State University of New York
State University of New York: Atomic Electron Configuration
This simulation displays the electron configurations of for the elements in relation to the element's position on the periodic table.
State University of New York
State University of New York: Electron Configuration of Ions
The following simulation displays electron configurations for elements in relation in relation to the element's position on the periodic table.
State University of New York
State University of New York: Electron Configurations
The following simulation provides an interaction with electron configurations.
Other
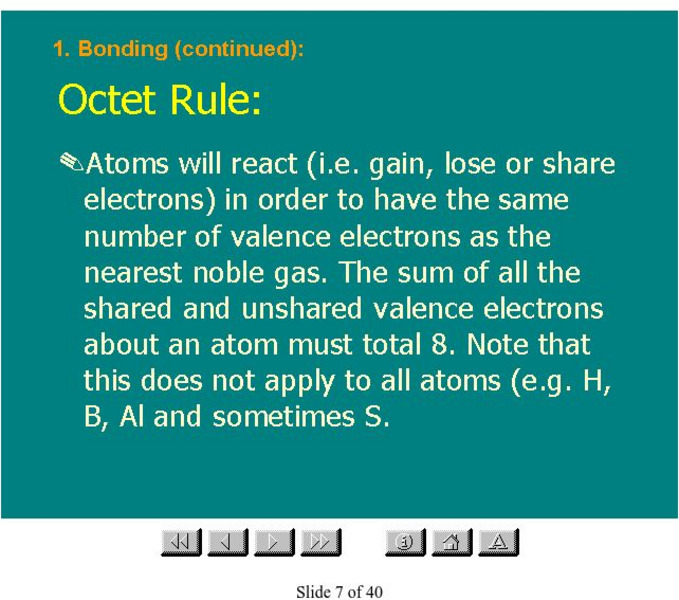
Organic Chemistry: Octet Rule
This slide presentation contains a description of the octet rule, as well as many related topics.