Science and Mathematics Initiative for Learning Enhancement (SMILE)
Smile: Electrochemistry
A teacher lesson plan which could be easily converted into an idea for a student project or presentation. This page describes activities in which the interconversion of chemical and electrical energy are investigated. Complete activity...
Wikimedia
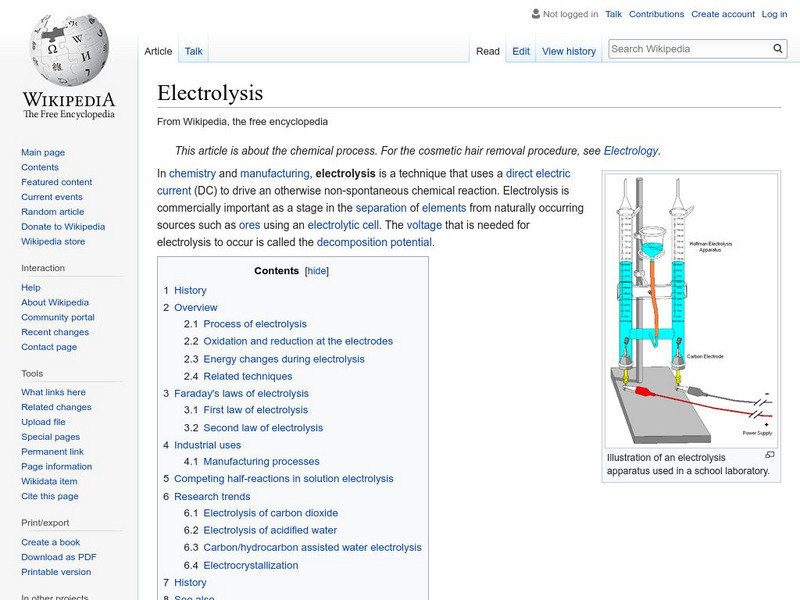
Wikipedia: Electrolysis
Wikipedia offers a short explanation of the how the electrolysis process works with links to some of the scientific pioneers of electrolysis.
Other
Lcs: Electrolysis of Silver Nitrate Solution
Complete directions for a teacher demonstration in which the electrolysis of a solution of silver nitrate is viewed under a microscope. Helpful tips and a diagram of the apparatus and set-up is provided. Easily adaptable as a student lab...
Other
Lcs: Experiments on the Electrolysis of Sodium Chloride
Complete directions several methods of conducting the electrolysis of sodium chloride solution. Helpful tips, discussion and a diagram of the apparatus and set-up is provided. Easily adaptable as a student lab or project.
Other
Case Western Reserve Univ.: Electrochemistry Dictionary
Dictionary containing definitions of words and phrases used in electrochemistry.
University of Illinois
University of Illinois Urbana Champaign: Chemistry Learning Center: Electrolysis of Water Using an Electrical Current
From the Chemistry Learning Center at the University of Illinois, this page explains the chemical changes occurring during a water electrolysis lab. Textual information is accompanied by photographs which clearly illustrate the changes.
ClassFlow
Class Flow: Reaction Types
[Free Registration/Login Required] Students cover the five general types of chemical reactions: synthesis, decomposition, combustion, single-displacement, and double-displacement. The flipchart concludes covering the reactions as the...
Hunkins Experiments
Hunkin's Experiments: How to Make Oxygen From a Battery
Hunkin's Experiments is a group of simple cartoon illustrations of scientific principles. Some would work well in the classroom, but others have little value beyond entertaining students. All of the projects are easy to do. This one...
Other
Nmsea: Electrolysis: Obtaining Hydrogen From Water
The New Mexico Solar Energy Association provides an article entitled, "Electrolysis: obtaining hydrogen from water - the basis for a solar-hydrogen economy". The article is medium size in length with pictures and charts throughout to help.
McMaster University
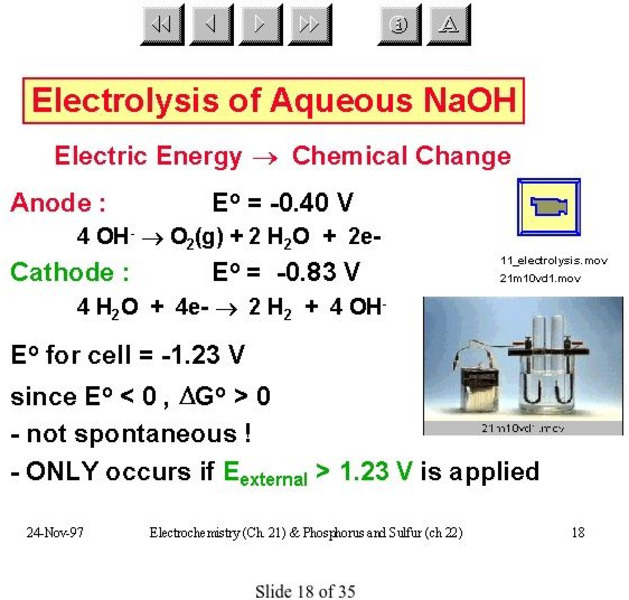
Mc Master University: Electrolysis of Aqueous Na Oh
Experimental setup and energy requirements for electrolysis of sodium hydroxide solution.
Science Struck
Science Struck: Understanding Decomposition Reaction
Explains what a decomposition is and gives examples of different types and their chemical equations.
PBS
Pbs Teachers: Scientific American: Journey to Mars: Out of Thin Air
Discuss facts about Mars and investigate scientists' efforts to create fuel from materials found in space. Perform a simplified method of aqueous electrolysis to split water into its two chemical elements using electric current from a...
Other
Miniscience: Electrolysis of Water
A simple science project designed to investigate electrolysis of water in a laboratory.
Mocomi & Anibrain Digital Technologies
Mocomi: Difference Between Electrolysis and Electroplating
Defines electrolysis and electroplating and their process and gives examples of uses.
MadSci Network
Mad Scientist Network: Nonproduction of Oxygen
From the Mad Scientist Network, this page (and its accompanying answer page) use a question and answer format to help explain the results of an electrolysis of water experiment. Helpful tips for performing such an experiment are given.