Curated OER
2007 U.S. National Chemistry Olympiad Local Section Exam
Sixty multiple choice questions cover the entire gamut of chemistry concepts. This is the local section of the U.S. National Chemistry Olympiad, where your chemistry candidates take a shot at entering the national competition. They...
Hazardous Waste Management Program
Molar Mass Determination from Freezing Point Depression
Let the data tell the story. A lab activity has pupils collect temperature data on solutes as they melt and freeze. They use their data to create a cooling curve and then calculate the molar mass using the freezing point depression.
DiscoverE
Ice Cream Special
We all scream for ice cream! Individuals create home-made ice cream in the classroom. This is a delicious way to show a real-world application of the freezing point depression to your class.
LABScI
Freezing Point Depression: Why Don’t Oceans Freeze?
Can you go ice fishing in the ocean? Learners examine the freezing point of different saltwater solutions. Each solution has a different concentration of salt. By comparing the freezing points graphically, they make conclusions...
University of Georgia
Freezing and Melting of Water
Examine the behavior of energy as water freezes and melts. An engaging activity provides a hands-on experience to learners. Collaborative groups collect data and analyze the graphs of the temperature of water as it freezes and then...
Curated OER
Gasoline Additive
Chemists consider a situation in which an ethanol producer needs to determine how much to add to t-butanol to prevent freezing during transport. They work in the laboratory to obtain the freezing point depression constant for the...
Curated OER
Ice Cream: a Taste of Science!!
Young scholars define the term solution. They explain conservation of energy and energy transfer as it relate to how the milk solution became ice cream. Students are able to explain freezing point depression.
Curated OER
WS 8.8 Molality and Colligative Properties
In this molality and colligative properties instructional activity, students determine the molality of solutions and they calculate freezing point depressions and boiling point elevations of solutions.
Curated OER
Solutions
Amateur chemists define and describe properties of solutions, compare solubilities, explain how solutes affect freezing and boiling points, describe acid and base properties, and more! This educational PowerPoint provides information and...
Curated OER
We All Scream for Ice Cream
Students investigate the freezing point depression of water while making ice cream. In this colligative properties lesson plan, students make ice cream using ice, salt and water to freeze milk and sugar. They measure the temperature of...
Curated OER
Carbohydrate Functionality - Sweeteners
In this carbohydrate worksheet, students conduct three experiments to determine the function of sweeteners. This worksheet has 3 short answer questions.
Curated OER
Quick Freeze (Demonstration)
Students witness an demonstration in which a bottle of club soda will go from a liquid to a solid when it is opened and the carbon dioxide is allowed to escape. This will help them understand that the freezing point of a solution will...
CK-12 Foundation
Ck 12: Colligative Properties and Molality
[Free Registration/Login may be required to access all resource tools.] Students distinguish between types of solutions such as electrolytes and nonelectrolytes and unsaturated, saturated, and supersaturated solutions.
CK-12 Foundation
Ck 12: Physical Science: Properties of Solutions
[Free Registration/Login may be required to access all resource tools.] How solutes affect solvents, the freezing point depression and boiling point elevation.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Colligative Properties of Solutions
This chemistry e-textbook provides students with AP-level reading and practice material on colligative properties of solutions.
CK-12 Foundation
Ck 12: Chemistry Simulation: Salty Roads
[Free Registration/Login Required] Explore how solutes can keep roads clear of ice in the winter by learning how different types and concentrations of a solute affect the freezing point of a solution.
PBS
Pbs Teachers: Going to Extremes: Frozen Alive
Explore survival strategies of animals in freezing climates. Investigate the role of glucose (sugar solution) when used as an antifreeze and the relationship between temperature and metabolism.
University of Maryland
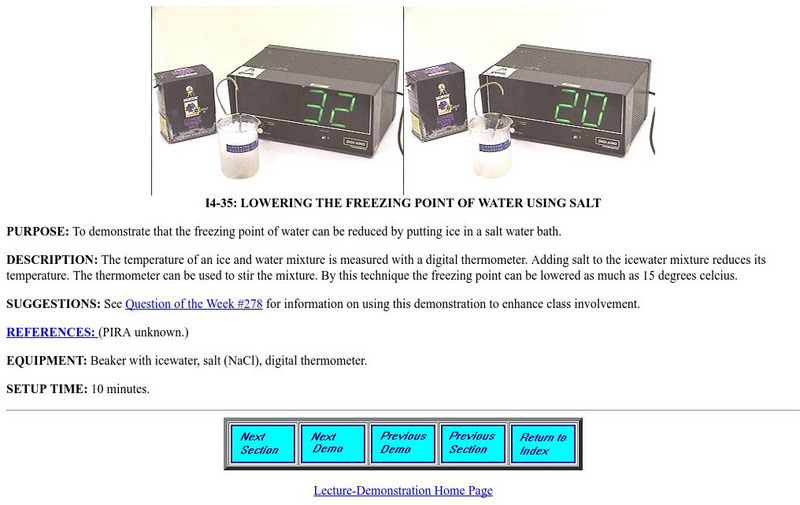
Lowering the Freezing Point of Water Using Salt
A page from the University of Maryland Physics Lecture Demonstration Facility. Provides directions for a teacher demonstration of the effect of an ionic solute upon the freezing point of water. Shows apparatus and set-up; provides...
Chem Tutor
Chem Tutor: Solutions
An in-depth look at solutions and their properties. Includes practice problems for calculating solution concentration.
Frostburg State University
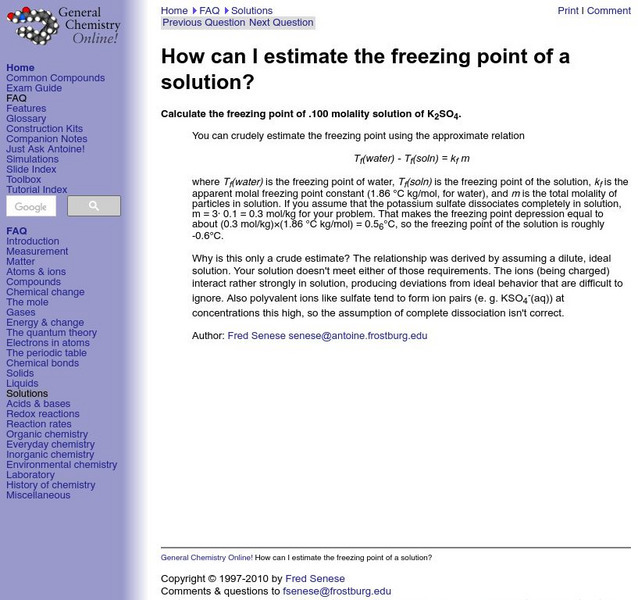
General Chemistry Online: Estimating Freezing Point
This site from the General Chemistry Online of the Frostburg State University provides informaiton on estimating freezing point of a solution from freezing point constant and molality.