Virginia Department of Education
A Crystal Lab
Young chemists grow ionic crystals, metallic crystals, and supersaturated crystals in three different lab experiments. Observing these under a microscope allows pupils to compare the various structures.
Virginia Department of Education
Matter and Energy: Equations and Formulas
Using simple materials, an informative lesson demonstrates the Law of Conservation of Matter and explains how to balance chemical equations. Young chemists perform experiments, analyze reactions, and balance chemical equations...
Curated OER
2007 U.S. National Chemistry Olympiad Local Section Exam
Sixty multiple choice questions cover the entire gamut of chemistry concepts. This is the local section of the U.S. National Chemistry Olympiad, where your chemistry candidates take a shot at entering the national competition. They...
Curated OER
Review of Ionic and Covalent Compounds and Transitioning from Ionic to Covalent Compounds
Here is a unique assigment: compare and contrast ionic and covalent compounds in an extensive data table and then analyze Lewis dot structures in antoher. Three columns are to befilled in: "characteristic or feature," "applies to ionic...
Curated OER
2001 U.S. National Chemistry Olympiad Part III
Here is a comprehensive method for assessing chemistry learners' knowledge; have them approach two laboratory problems, plan their methods of solving each, and then actually carry out the experiments to find the answers. Both the...
Curated OER
2009 U. S. National Chemistry Olympiad - Local Section Exam
Here is a copy of a past national challenge exam that you can use in your general chemistry course as a unit or semester review. Sixty multiple-choice questions query learners on properties of matter, stoichiometry, reactions, and...
Curated OER
Properties of Ionic and Covalent Compounds
Test six various reagents for their ability to conduct electricity. Cooperating chemists research whether each is ionic or covalent and discover the relationship between bonding and conductivity. This laboratory exercise would be an...
Creative Chemistry
Common Ions and Formulae of Ionic Compounds
This handout, produced in the UK, contains a chart of cations and anions. It explains how ionic compounds are formed and named. This concise and attractive handout can be helpful as a reference for your chemistry apprentices.
Learning Games Lab
Nitrogen in Fertilizer
Nitrogen is an essential element for productive farming. An interactive lesson explores the chemical makeup of different fertilizers and their corresponding nitrogen content. The interactive challenges individuals to complete...
College Board
2015 AP® Chemistry Free-Response Questions
More than 80,000 scholars earned college credit for Chemistry with the AP exam in 2015. The College Board released the free-response questions covering topics, including moles, that often confuse scholars. They also released example...
College Board
2016 AP® Chemistry Free-Response Questions
The College Board published the mean score on the 2016 AP Chemistry exam as a 2.69, but a minimum of a three is required to earn college credit. Help pupils study for the upcoming exam with actual test questions, sample answers, and...
Pingry School
Solubility Product of an Ionic Compound
How do scientists determine when a solution is fully saturated? Scholars address the topic as they observe patterns of precipitation in various concentrations of ions. Using a well plate, pipette, and common chemicals, they collect data...
Royal Society of Chemistry
Ionic Formulae 1
The ionic formula for banana would be BaNa2. Scholars work their way through four puzzles reviewing ionic formulas. Each puzzle requires scientific knowledge as well as logic and problem-solving skills.
Royal Society of Chemistry
Ionic Formulae 2
Scientists call ions with a negative charge anions. Scholars work through one matching puzzle and four logic puzzles reviewing the chemical formulas for anion ions. Each level increases in difficulty and solidifies the concept in pupils'...
Royal Society of Chemistry
Common Compounds
Can your young chemists identify the most commonly used chemicals in the lab? Introduce the class to the go-to substances in most middle and high school chemistry experiments with an interactive. The resource offers timely feedback as...
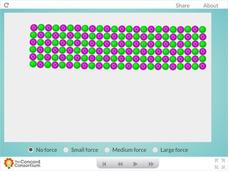
Concord Consortium
Ceramic Forces
Why are bricks more likely to break than bend? Young science scholars peer inside a ceramic block and examine the effects of downward force at the molecular level. Learners can apply three different levels of force before observing their...
CK-12 Foundation
Mineral Formation: Evaporating Lake
Get crazy for crystals! Junior geologists learn the secrets of crystal formation through lecture, reading, and examples. Other topics include common ionic compounds found in fresh and salt water, the effects of location on forming...
Science Geek
Ionic Bonding
Here's a presentation that answers the age-old question of the covalent bond to the ionic bond, "Why won't you share?" Included is information about covalent and ionic bonds, the octet rule, ionic compounds, cations versus anions, and...
Science Geek
Ionic Compound Formulas
By contrasting cations and anions, this presentation shows how to predict ionic charges by periodic groups. The slides conclude with a few guided practice problems for writing ionic compound formulas.
Virginia Department of Education
Formulas and Percent Compositions of Ionic Compounds
Try not to blind anyone with science by following the safety rules. The lesson encourages scholars to form an ionic compound from magnesium and chlorine. Then they determine the empirical formula and determine the mole ratio and percent...
It's About Time
Chemical Names and Formulas
Abracadabra! Provide your class with the tools to perform a chemical "magic show" as they predict the charges of various ions, determine ionic compound formulas, and make observations to determine when a chemical reaction between...
National Science Teachers Association
Ionic Molecular
Twelve compounds are named and each of their chemical formulas are printed on individual cards. Chemistry pupils place each beneath an Ionic or Molecular card, depending on which type of compound is represented.
Curated OER
Ionic Nomenclature
One document contains five different worksheets for practice naming and writing formulas for ionic compounds. The first is particularly notable, as it systematically walks beginning chemists through the process of using the periodic...
Creative Chemistry
Common Ions and Formulae of Ionic Compounds
This is not a learning exercise per se but a reference sheet for your chemistry class. It lists cations and anions, their chemical symbols, and their net charges. Also included is a thorough explanation of combining these ions to form...