Curated OER
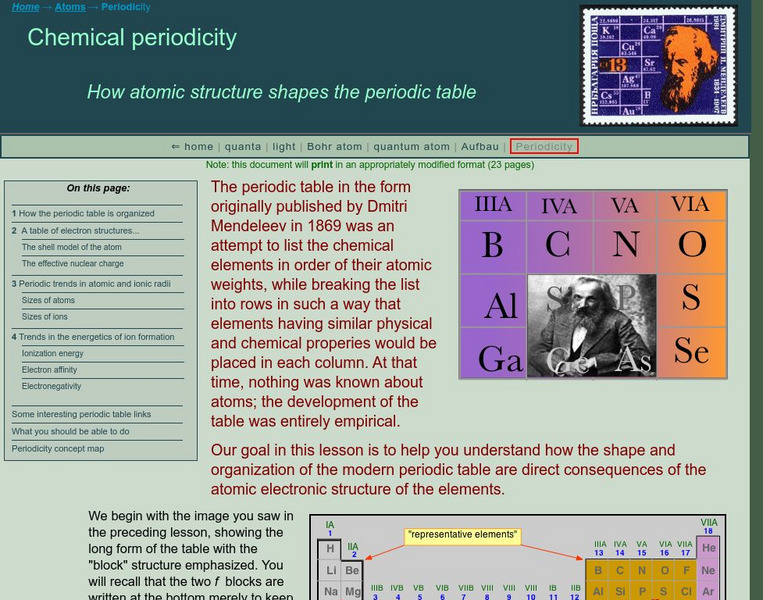
Electromagnetic Radiation and the Bohr Atom
In this light worksheet, students calculate the frequency and wavelength. Students practice applying Planck's constant and determining the energy of a photon. This worksheet has 9 problems to solve.
Shodor Education Foundation
Shodor: Background Reading for Ionization Energy
A very complete lesson on ionization energy, including tables and charts to illustrate the ideas presented in the text.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
Other
University of South Carolina: Ionization Energy
First slide in a thorough presentation of ionization energy from a trend to an explanation of isoelectric series. Gives you practice problems as well.
CK-12 Foundation
Ck 12: Periodic Trends
[Free Registration/Login may be required to access all resource tools.] In the following online tutorail students will use the Periodic Table to identify and explain periodic trends, including atomic and ionic radii, electronegativity,...
CK-12 Foundation
Ck 12: Trends in the Periodic Table
[Free Registration/Login may be required to access all resource tools.] Students investigate specific properties that can be predicted by an element's position on the periodic table. Additionally, they will look at the formation of ions,...
Khan Academy
Khan Academy: Photoelectron Spectroscopy
An explanation of how photoelectron spectroscopy can be used to detect electron structures in atoms or molecules.
Simon Fraser University
Chem1 Virtual Textbook: Spectrum of the Hydrogen Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the hydrogen atom and its relation to spectrum. Included in the discussion is information on the Bohr model...
Simon Fraser University
Chem1 Virtual Textbook: Periodic Trends in Ion Formation
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses electron affinity and ionization energy in relation to ion formation. Charts and graphs are included as well.
Michael Blaber, PhD
Florida State Univ.: Periodic Properties of the Elements: Ionization Energy
Exceptional article from the Florida State University that will take you from start to finish. This article will answer all your questions about ionization energy as both a concept and trend.
Clackamas Community College
Clackamas Community College: Ionization Energy
Detailed explanation of ionization energy with charts. Also gives practice problems with explanation of the answers.
Florida State University
Florida State University: Magnet Lab: Atmospheric Pressure Photoionization (Appi)
In the APPI technique, UV light photons are used to ionize sample molecules.
Sophia Learning
Sophia: Ionization Energy: Lesson 1
This lesson will define ionization energy. It is 1 of 3 in the series titled "Ionization Energy."
Sophia Learning
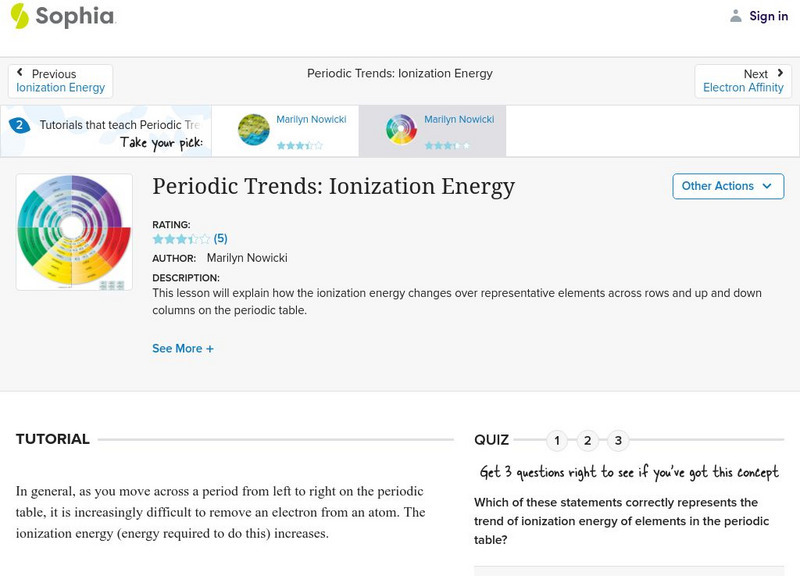
Sophia: Periodic Trends: Ionization Energy: Lesson 3
This lesson will explain how the ionization energy changes over representative elements across rows and up and down columns on the periodic table. It is 3 of 3 in the series titled "Periodic Trends: Ionization Energy."
National High Magnetic Field Laboratory
Magnet Academy: Deionization
The magnets here at the lab can generate massive amounts of heat. To cool them off, we need massive amounts of water. But first, we have to take the ions out.
CK-12 Foundation
Ck 12: Chemistry: Periodic Trends: Ionization Energy
[Free Registration/Login may be required to access all resource tools.] Explains ionization energy, trends in ionization energy, and electron shielding.
CK-12 Foundation
Ck 12: Chemistry: Electron Shielding
[Free Registration/Login may be required to access all resource tools.] Covers electron shielding, s orbital influence on shielding, paired electron spin and shielding.
Web Elements
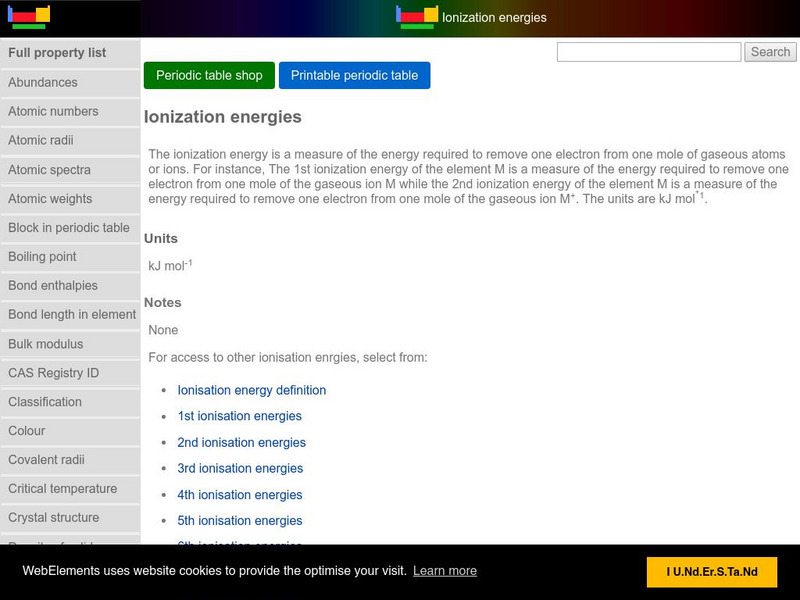
Web Elements Periodic Table: Ionization Energy
This WebElements site allows you to create a periodic table that shows the relative ionziation energies of all elements. Great visuals!!
CK-12 Foundation
Ck 12: Chemistry: Periodic Trends: Ionization Energy
[Free Registration/Login may be required to access all resource tools.] Explains ionization energy, trends in ionization energy, and electron shielding.
Frostburg State University
General Chemistry Online: The Periodic Table
This is a teacher's companion guide with lesson plans for the periodic table. It includes learning objectives, lecture notes, links to related sites, answers to frequently asked questions, and a glossary of related terms.
Oklahoma State University
Oklahoma State University: Ionization Energy Trends
Site that discusses trends in ionization energy across and down the periodic table. Apparent contradictions are explained using electron configurations.
Georgia State University
Georgia State University: Hyper Physics: Ionization Energies
A concise description of ionization energy, and a line graph that plots the ionization energies of the first 88 elements vs. the atomic numbers.
Upper Canada District School Board
Tom Stretton's Chemistry Pages: Ionization Potential and Electronegativity
Deepen your understanding of ionization potential and electronegativity in this illustrated presentation.
Sophia Learning
Sophia: Ionization Energy: Lesson 2
This lesson will define ionization energy. It is 2 of 3 in the series titled "Ionization Energy."