Sophia Learning
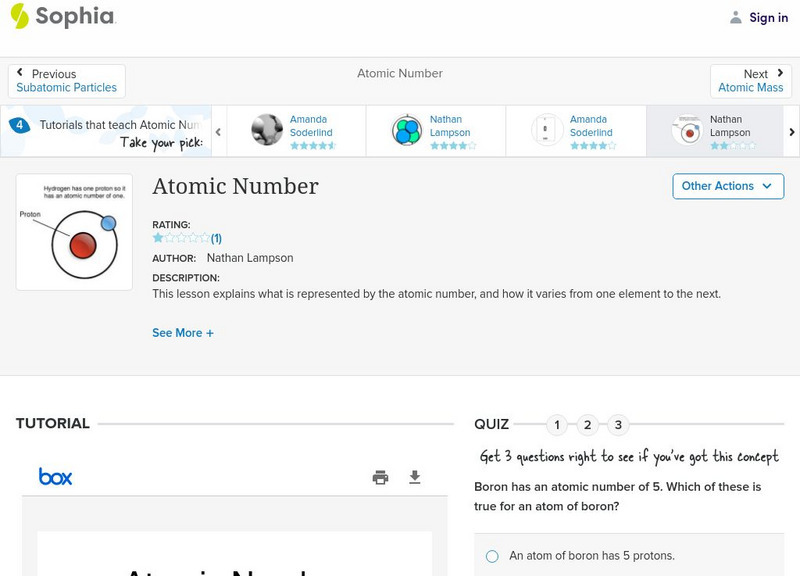
Sophia: Atomic Number: Lesson 6
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 6 of 7 in the series titled "Atomic Number."
Sophia Learning
Sophia: Atomic Mass: Lesson 2
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 2 of 4 in the series titled "Atomic Mass."
Sophia Learning
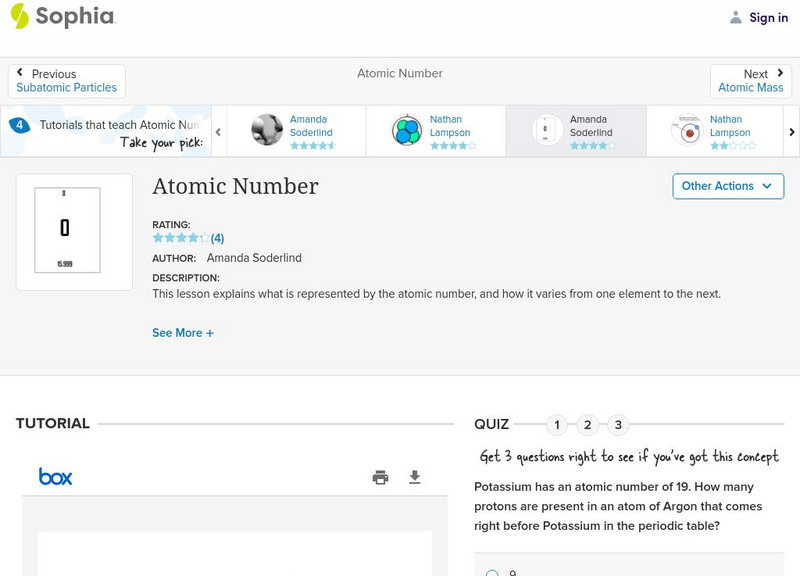
Sophia: Atomic Number: Lesson 5
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 5 of 7 in the series titled "Atomic Number."
ClassFlow
Class Flow: Element Math
[Free Registration/Login Required] In this flipchart students determine the number of protons, neutrons, and electrons of elements essential to life. They use Activotes to check knowledge gained. Students create a Bohr Model.
ClassFlow
Class Flow: Intro to Atoms
[Free Registration/Login Required] This flipchart was converted from Power Point and is used to introduce the history and concept of the Atom.
Science Struck
Science Struck: How to Find Protons, Neutrons and Electrons
Brief explanations of how to determine how many protons, neutrons, and electrons are in an element.
Science Struck
Science Struck: What Makes Up an Atom?
Describes the structure of an atom and the characteristics of the electrons, neutrons, and protons inside it. Includes some interesting facts about atoms.
California State University
California State University: Proton, Electron, Neutron
An interesting and useful tool to practice recognition/calculation of atomic number, mass, and number of neutrons, electrons, etc. Can be used with all elements.
Mocomi & Anibrain Digital Technologies
Mocomi: Structure of an Atom
An atom is made of three parts - protons, neutrons, and electrons. Here you can explore these different parts.