PBS
Data Plots of Exoplanet Orbital Properties
Scientists discovered the first exoplanet in 1995 and by early 2018, they confirmed the existence of more than 3,700—that's a lot of data! As part of the PBS 9-12 Space series, scholars interpret data about exoplanets. They compare...
Royal Society of Chemistry
Sub-shells
Is your class in a quandary over quantum numbers? Change things up by adding games to the mix! Science scholars discover the shape, number of electrons, and number of orbitals in the s, p, and d sub-shells using an interactive.
Royal Society of Chemistry
Shapes of Molecules—Hybrid Orbitals
Take your chemistry class' knowledge of molecular geometry to the next level! Introduce orbital hybridization with a series of related games. Individuals complete a data table in the first activity, then solve Sudoku-like puzzles using...
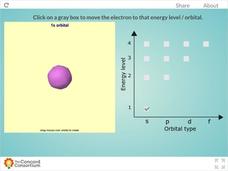
Concord Consortium
Energy Levels of a Hydrogen Atom
Tired of blowing up countless balloons to illustrate orbital shapes around an atom? Give your lungs a break and use an interactive instead! Learners observe s, p, d, and f orbitals through the first four energy levels using hydrogen as a...
CK-12 Foundation
Orbital Motion
Why do planets orbit the sun in ellipses when moons orbit their planet in circles? Pupils control the semi-major axis, eccentricity of the orbit, and position angle. The resulting orbital appears with the related force vectors as...
McGraw Hill
The Bohr Atom
Elements don't have fingers, but they have fingerprints! An interactive simulation gives young scientists the opportunity to study orbital changes of an atom and the corresponding spectrum reading. They realize how each atom has a...
National Institute of Open Schooling
Atomic Structure
Learners explain historical findings such as Rutherford and Bohr's contributions, explain wave particle duality, and formulate Heinsenberg's uncertainty principle. They also draw s, p, and d orbitals, explain more historical findings,...
National Institute of Open Schooling
Chemical Bonding
Name is Bond, covalent bond. Through readings and answering questions, classes explore the different types of chemical bonds, their characteristics, valence shell electron pair repulsion theory, and atomic orbitals.
Mr. Jones's Science Class
Parts of the Atom
Up and atom! After labeling the parts of an atom, young chemists answer 16 diagram-based questions that deal with protons, neutrons, electrons, and atomic number.
Virginia Department of Education
Planet Line-Ups
Should Pluto be considered a planet or a dwarf planet? Scholars research planets in our solar system to understand their similarities and differences. It also includes memory activities related to the order of the planets.
Curated OER
Organic Chemistry Problem Set Exam 1
Though there are technically only 13 questions on this exam, they take up six pages and make a thorough assessment of organic chemistry principles. There are plenty of diagrams to label or complete. Emission spectra are displayed for...
Curated OER
Unit 2 ~ Atomic Structure
As an atomic structure reference and review tool, this handout fits the bill. The first page provides definitions and tables of orbitals, electrons, and energy levels. The second page is an opportunity to practice determining numbers of...
Curated OER
Organic Chemistry 231, Martin Larter, Exam 1
If you need a straightforward and comprehensive organic chemistry exam, check this one out. Chemistry pupils identify functional groups in molecule diagrams, draw a Lewis structure, fill in a table about molecular shape, predict boiling...
Spark Notes
Review of Chemical Bonding: Review Test
This is an online exercise in which chemistry learners answer a series of multiple choice questions about bonding. Topics addressed include ionic and covalent bonds, electronegativity, ions, valence electrons, resonance structure, and...
Curated OER
The Atom Board - Making Atoms
In this atom worksheet, chemists learn how to determine the number of protons, neutrons and electrons in an atom using the atomic mass and atomic number. They complete a chart of the subatomic particles and use marbles to represent...
Curated OER
An Electron's Address
Where does an electron reside? Chemistry scholars determine an electron's "address," that is, what orbital it can be found in. This resource is both instructional and practical, providing a thorough explanation of energy levels and...
Curated OER
Elements, Atoms, Ions, and the Periodic Table
This PowerPoint provides a complete recap of all the information needed for a unit on elements and the periodic table. The seventy slides cover the basic notation of elements, energy levels and ionization energies. The trends in the...
Curated OER
The Periodic Table
A huge collection of slides introduces chemistry learners to the periodic table of elements, electron configuration, and electronegativity. It opens with the history of today's periodic table, and then details the arrangement. After...
Curated OER
Patterns in Electron Configuration
A periodic table of elements and ten accompanying questions comprise this chemistry handout. Learners survey the table to discover the relationship between group columns and valence electrons. They also associate energy levels to the...
Curated OER
Essential Elements
A color-coded periodic table identifies organic elements, major minerals, and trace elements. Oxidation states are highlighted and types of chemical bonding are annotated. The electron energy level chart is explained. Though not all of...
Curated OER
Location of Electrons
In this location of electrons activity, students read about the quantum mechanical model of the atom and the location of electrons. They complete a table given eleven elements with the sublevel notation, the Bohr notation and the orbital...
Curated OER
Configure Your Electron Notes
In this electron configuration worksheet, students fill in a table with the number of electrons, the number of orbitals, the type of orbitals and the total number of electrons per orbital in each principle energy level. They then write...
Curated OER
Where are the Electrons?
In this electrons worksheet, students complete a graphic organizer to show the pattern of the principal energy levels, number of orbitals, and electrons in each sublevel of atoms.
Curated OER
Electron Structure of the Atom
In this atoms worksheet, students determine the energy difference between levels and draw diagrams of the different types of orbitals. Students determine the ground state electron configuration for given elements. This worksheet has 14...