Curated OER
Swinging Electrons
Young scholars illustrate paramagnetic and diamagnetic differences due to the unfilled and filled valence shells. They prepare saturated solutions and place into test tubes.
Georgia State University
Georgia State University: Hyper Physics: Pauli Exclusion Principle
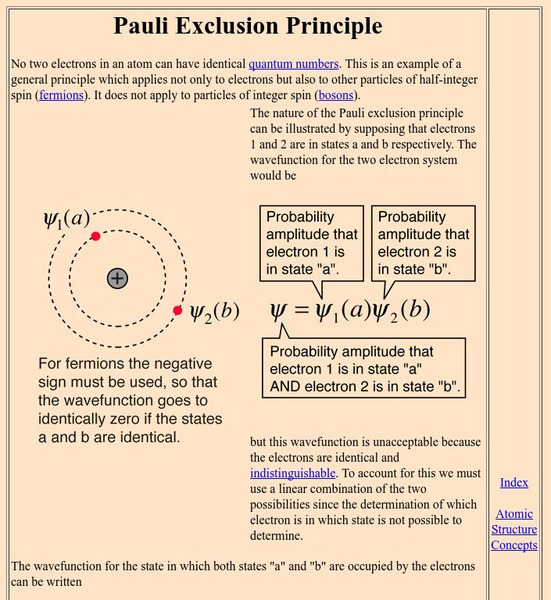
An explanation of the Pauli Exclusion Principle in mathematical terms.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Electron Clouds and Energy Levels
An explanation of the different types of atomic orbitals, how they are filled according to the Pauli Exclusion Principle, and how many electrons can fit in each electron shell.
Simon Fraser University
Chem1 Virtual Textbook: Bonding in Metals and Semiconductors
Acting as an overview from the General Chemistry Virtual Textbook, this site explores chemical bonding in relation to metals and semiconductors. Topics covered include molecular orbitals in metals, band structure in metals, and more.
Michael Blaber, PhD
Florida State University: Electronic Structure of Atoms: Electron Configurations
Florida State University provides this article on electronic configurations of atoms and Hund's rule.
CK-12 Foundation
Ck 12: Electron Arrangement in Atoms
[Free Registration/Login may be required to access all resource tools.] In the following online tutorail students will learn how to express the arrangement of electrons in atoms through electron configurations and Lewis valence electron...
Khan Academy
Khan Academy: The Quantum Mechanical Model of the Atom
An explanation using quantum mechanics to describe the atom.
Khan Academy
Khan Academy: Practice Writing Electron Configurations
Choose the element that matches the electron configuration.
Khan Academy
Khan Academy: Bond Hybridization
Practice determining the hybridization for atoms in covalent compounds. Use the interactive scratch pad to work out the problems.
Texas Education Agency
Texas Gateway: Electron Configuration
This tutorial explores electron configuration. Learn about electron configuration through interactive activities, quizzes, and videos.
CK-12 Foundation
Ck 12: Orbitals
[Free Registration/Login may be required to access all resource tools.] A collection of learning opportunities about electron orbitals. Includes videos, activities, discussion questions, and quizzes.
Other
University of Texas: Tabled Discussion
At this University of Texas site, atomic orbital occupancy, quantum numbers, the Aufbau Principle, and periodic trends are described in detail.
Davidson College
Davidson College: Visualization of Atomic Orbitals: Hybrid Orbitals
Explains the creation of hybrid orbitals and presents their electron density plots and energy diagrams. Requires Java.
Georgia State University
Georgia State University: Hyper Physics: Hund's Rules
A complete overview of Hund's rules including exceptions.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Complete Electron Configurations Help
This section from the online textbook "An Introduction to Chemistry", provides some helpful hints to writing complete electron configurations. Three ways to predict the order of filling electron orbitals are given.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Multi Electron Atoms: Audio Book
This audio book, narrated by author Mark Bishop, describes how to write regular and abbreviated electron configurations, and how to draw orbital diagrams. Also find links to animations, tutorials, power points, and chapter maps about...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Complete Electron Configuration
Test your knowledge of common atoms and their corresponding electron configurations. In this interactive exercise, you will find out how much you really know about the orbitals of electrons.
Oklahoma State University
Osu: Chemical Bonding Content in a Nutshell
An overview of chemical bonding and how valence electrons play a major role in these processes.
Simon Fraser University
Chem1 Virtual Textbook: The Quantum Numbers
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses quantum numbers of electrons in atoms including topics such as principal quantum number and orbitals of the...
Simon Fraser University
Chem1 Virtual Textbook: Molecular Orbitals
Acting as an overview from the General Chemistry Virtual Textbook, this site explores the topics related to molecular orbitals including bonding and antibonding MOs, molecular orbital diagrams, sigma and pi orbitals, and more.
Simon Fraser University
Chem1 Virtual Textbook: Sigma and Pi Orbitals
Acting as an overview from the General Chemistry Virtual Textbook, this site explores sigma and pi orbitals covering topics such as the importance of direction, splitting patterns for the second row diatomics, Dicarbon, Dioxygen, and more.
Purdue University
Purdue University: Quantum Numbers & Electron Configurations
This site from the Purdue University provides quantum numbers explained in detail and shown how they specify the atomic orbital of each electron and their energy levels. Electronic configurations are explained. Learning exercises with...
Purdue University
Purdue University: Orbital Shells
This site from the Purdue University provides an overview of atomic orbitals and how quantum numbers specify these orbitals. Includes a model that can be rotated and examined in 3D. Includes learning exercises and answers.