BiologyWise
Biology Wise: An Overview of the Difference Between Nadh and Nadph
NADH and NADPH are enzymes involved in cellular processes. Explains how NADH participates in catabolic reactions where energy is released and NADPH is involved in anabolic reactions where energy is consumed. Their functions and chemical...
TED Talks
Ted: Ted Ed: Is Fire a Solid, a Liquid, or a Gas?
Elizabeth Cox illuminates the science behind fire.
PBS
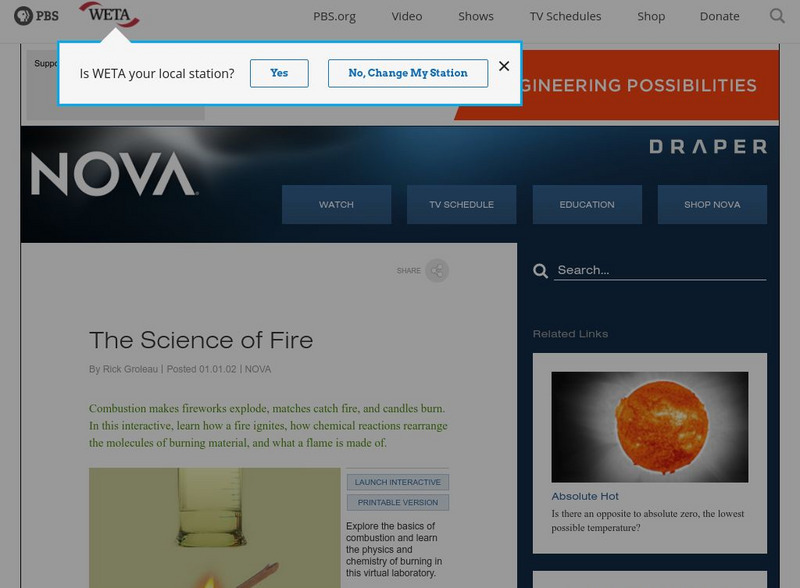
Pbs: The Science of Fire
A virtual experiment bringing you to the center of combustion! Understand how fire ignites, what makes a flame, and how molecules rearrange themselves in chemical reactions. This interactive activity gives students four different...
Khan Academy
Khan Academy: Pyruvate Oxidation
How pyruvate from glycolysis is converted to acetyl CoA so it can enter the citric acid cycle. Pyruvate is modified by removal of a carboxyl group followed by oxidation, and then attached to Coenzyme A.
Khan Academy
Khan Academy: Redox Reactions Questions
This is a 10-question exercise/quiz pertaining to redox reactions.
Khan Academy
Khan Academy: Biological Oxidation of Alcohols
Article takes a look at how the body processes alcohol.
Georgia State University
Georgia State University: Hyper Physics: Voltaic Cells
Georgia State University provides a technical discussion on how voltaic cells produce an external electric current. Equations for the reactions that occur are given and discussed.
Science and Mathematics Initiative for Learning Enhancement (SMILE)
Smile: Electrochemistry
A teacher lesson plan which could be easily converted into an idea for a student project or presentation. This page describes activities in which the interconversion of chemical and electrical energy are investigated. Complete activity...
Simon Fraser University
Chem1 Virtual Textbook: The Fall of the Electron
With an overview of topics related to chemical equilibrium, this site provides a foundation to a study of thermodynamics and electrons. Included in the discussion is information on oxidation-reduction reactions.
Wikimedia
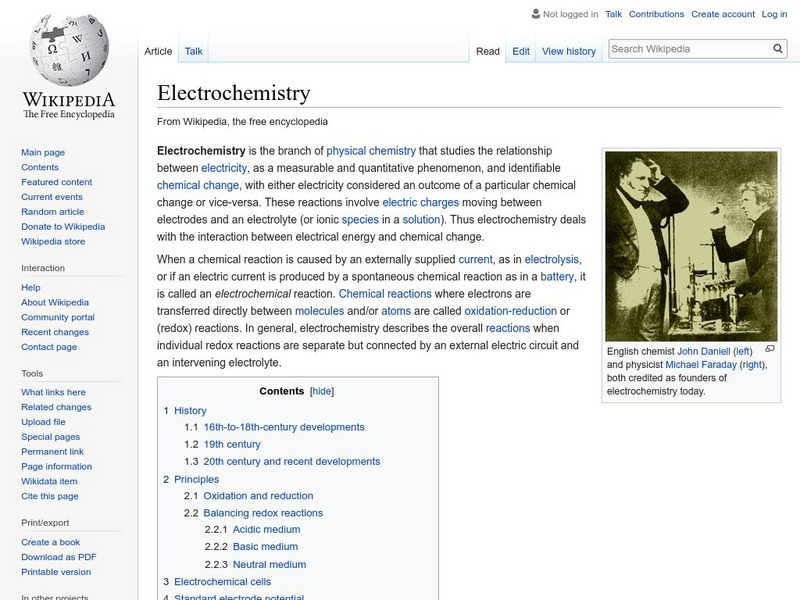
Wikipedia: Electrochemistry
This site from Wikipedia enyclopedia provides this discussion over electrochemistry details what the oxidation state of an ion is; what oxidation and reduction is; what redox reactions are; and the difference between spontaneous and...
Wikimedia
Wikipedia: Aldehyde
This encyclopedia entry for aldehyde compounds features naming convention, manufacture, and examples of aldehydes.
Other
The Science House: Combustion
In this experiment, students will observe that the weight of the product of combustion is greater than that of the starting material. Teacher's notes address the key concepts of this experiment.
Other
Lcs: Electrolysis of Silver Nitrate Solution
Complete directions for a teacher demonstration in which the electrolysis of a solution of silver nitrate is viewed under a microscope. Helpful tips and a diagram of the apparatus and set-up is provided. Easily adaptable as a student lab...
Other
Lcs: Experiments on the Electrolysis of Sodium Chloride
Complete directions several methods of conducting the electrolysis of sodium chloride solution. Helpful tips, discussion and a diagram of the apparatus and set-up is provided. Easily adaptable as a student lab or project.
Educaplus (Jesús Peñas Cano)
Educaplus: Reacciones Redox [In Spanish]
Try to adjust the different oxidation-reduction reactions.
Other
Aus E Tute: Galvanic (Voltaic) Electrochemical Cells
Animated demonstration of how a galvanic cell works. Demo defines oxidation and reduction and shows how the electric circuit must be completed with a salt bridge to allow the electrons to flow from the anode to the cathode.
American Geosciences Institute
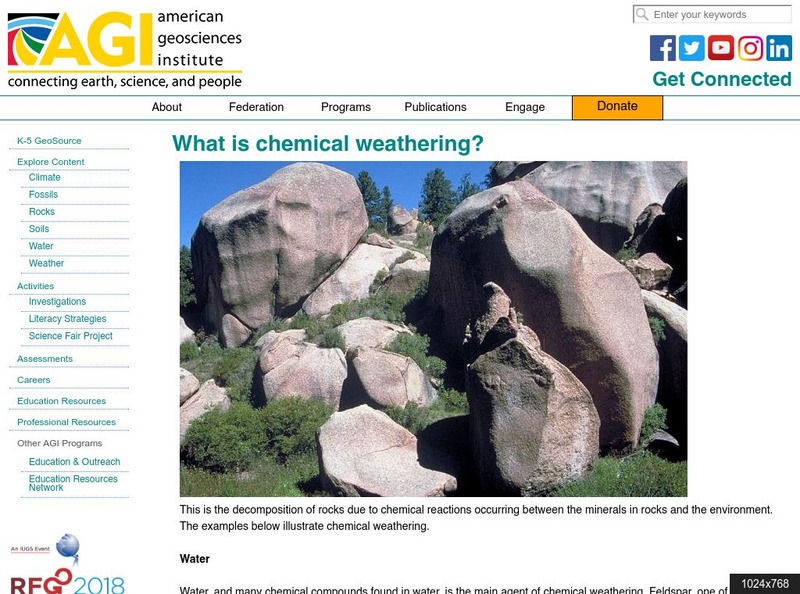
American Geosciences Institute: What Is Chemical Weathering?
Learn about the chemical weathering processes that break down rocks.
ClassFlow
Class Flow: Cellular Respiration
[Free Registration/Login Required] This is a comprehensive, engaging overview of catabolic pathways that yield energy by oxidizing organic fuel. Students explore redox reactions, oxidation, reduction, and preview the stages of cellular...
Simon Fraser University
Chem1 Virtual Textbook: Latimer Diagrams
As part of the General Chemistry Virtual Textbook, this site examines a variety of topics related to electrochemistry. This site in particular examines the Latimer diagrams, which is a means of "comparing the standard electrode...
Science Struck
Science Struck: Chemical and Physical Properties of Magnesium
Presents facts about Magnesium, descriptions of its physical and chemical properties, and examples of its uses.
MadSci Network
Mad Scientist Network: Nonproduction of Oxygen
From the Mad Scientist Network, this page (and its accompanying answer page) use a question and answer format to help explain the results of an electrolysis of water experiment. Helpful tips for performing such an experiment are given.
Curated OER
Georgia State University: Voltaic Cells
Georgia State University provides a technical discussion on how voltaic cells produce an external electric current. Equations for the reactions that occur are given and discussed.
Curated OER
Georgia State University: Voltaic Cells
Georgia State University provides a technical discussion on how voltaic cells produce an external electric current. Equations for the reactions that occur are given and discussed.













![Educaplus: Reacciones Redox [In Spanish] Activity Educaplus: Reacciones Redox [In Spanish] Activity](https://content.lessonplanet.com/knovation/original/125127-45feec0cd3a32821aea0d12f99826ac7.jpg?1661774229)