Michael Blaber, PhD
Fsu: Electronic Structure of Atoms: Quantum Mechanics & Atomic Orbitals
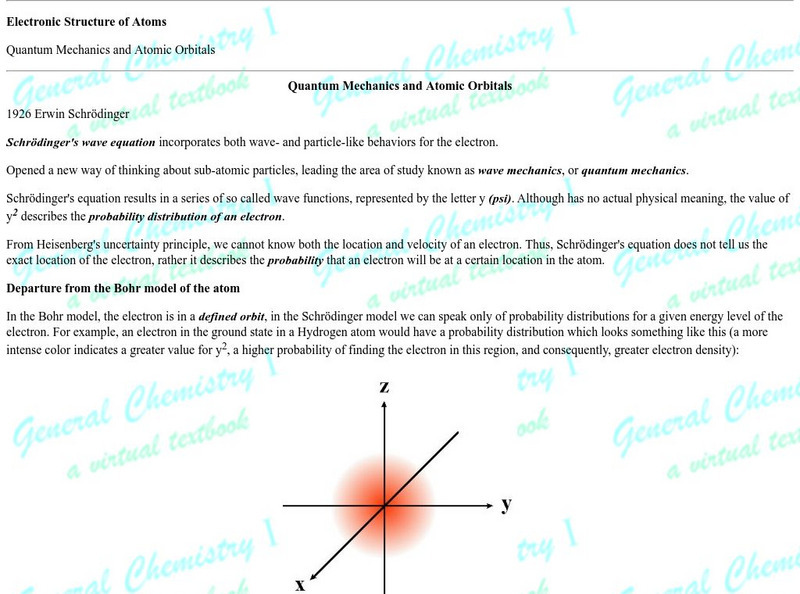
This article is provided by Florida State University. Schroedinger wave equation is explained and how quantum numbers are used to describe the energy level of any electron in an atom. The relationship of atomic orbitals to quantum...
American Association of Physics Teachers
Com Padre Digital Library: Open Source Physics: Rectangular Well Superposition
A simulation that displays the 2D evolution of the position-space of a wave in an infinite 2D rectangular well.
State University of New York
State University of New York: Atomic Orbital Energies
This simulation explores the trends observed in orbital energies for the main group elements of atomic orbital energies.
Virginia Tech
University of Vermont: Quantum Numbers
This chart highlights the characteristics of the first through fourth electron orbitals in quantum numbers. Also includes information about the Pauli Exclusion Principle.
Concord Consortium
Concord Consortium: Molecular Workbench: Atomic Orbitals
View the approximate locations of electrons in valence shells 1s through 4f.
Georgia State University
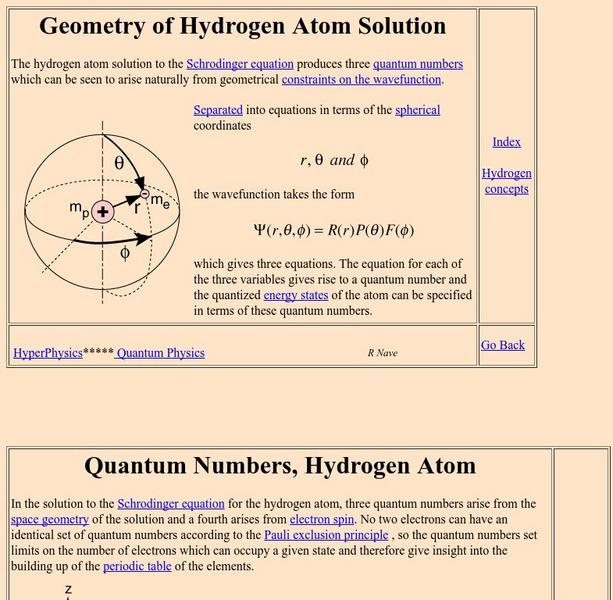
Georgia State University: Hyper Physics: Quantum Numbers, Hydrogen Atom
This tutorial contains links to explanations of the four different quantum numbers (principal, orbital, magnetic, and spin). Equations for each are provided.
Simon Fraser University
Chem1 Virtual Textbook: Electron Spin and the Exclusion Principle
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses electron spin with related topics, such as the Pauli exclusion principle, quantum numbers, and more.
Sophia Learning
Sophia: Quantum Numbers: Lesson 2
This lesson will introduce quantum numbers, their symbols, and what they represent. It is 2 of 3 in the series titled "Quantum Numbers."