Curated OER
Chemistry
In this chemistry worksheet, students describe an atom, its parts, the charges of these parts, and atoms life functions. Then they explain what the octet rule states for the placements of electrons around the nucleus of an atom. Students...
Curated OER
Osmosis
In this biology worksheet, students determine how many solvent and solute molecules there are in the red blood cell and the IV fluid. Then they determine whether the IV fluid is hypertonic, hypotonic, or isotonic to the red blood cell.
Curated OER
Bacterial Movement Through Water Part 2- Dilution/Powers of Ten
Students make dilutions using dulution trays. They keep track of their dilutions and the concentrations of solute, to parts per million (ppm) and parts per billion (ppb), in order to have an comprehension of the terms. Students make...
Curated OER
More on Temperature and Solubility
Learners discover how temperature effects the solubility of solutes by experimenting with a range of temperatures. They develop skills for observing, inferring, measuring, comparing and contrasting.
Curated OER
Study the Fizz
Students hypothesize about the amount of carbon dioxide in soda bottles. In this chemistry lesson plan, students design an experiment to solve the problem. They share findings in class.
Curated OER
Solubility
In this solubility worksheet, students determine which solute will be more soluble in the solvent listed. Students complete 7 matching and 3 problems to solve.
Curated OER
Colligative Properties Worksheet
In this solutions worksheet, learners determine the boiling points and melting points of solutions. Students calculate the effective molality of a solute. This worksheet has five problems to solve.
Curated OER
Solutions Quiz Review Sheet
In this solutions activity, students use a phase diagram to determine the boiling point and molality of the solution. Students determine the electrical conductivity of a saturated solution. This activity has nine problems to solve.
Curated OER
Working with Solutions
In this solutions worksheet, students review how molarity is calculated and how to prepare a dilute solution. This worksheet has 5 problems to solve.
Curated OER
Solution Concentration
In this solutions worksheet, students define solute and solvent. Students calculate the concentration of solutions. This worksheet has 4 short answer questions and 4 problems to solve.
Curated OER
Solvents
In this solvents pre-lab activity, students describe the difference between a polar and non-polar solvent and also the dangers associated with hexanes and iodine. This activity has 12 short answer questions.
Curated OER
How Can Mixtures Be Separated?
In this mixtures worksheet, students brainstorm the problems and possible solutions of how to separate mixtures. This worksheet is a graphic organizer.
Curated OER
Science: Quick Freeze
Learners observe an experiment of freezing point depression using club soda. Through observation, they note that the carbon dioxide molecules disrupt the capacity of the water molecules to solidify. Precautions must be taken to assure...
Curated OER
Teaching about Conductivity
Students explore conductivity and productivity in aquatic systems.
Vision Learning
Visionlearning: Physical States and Properties: Properties of Liquids
Read about the many properties of liquids. Take an assessment when finished.
CK-12 Foundation
Ck 12: Physical Science: Properties of Solutions
[Free Registration/Login may be required to access all resource tools.] How solutes affect solvents, the freezing point depression and boiling point elevation.
Frostburg State University
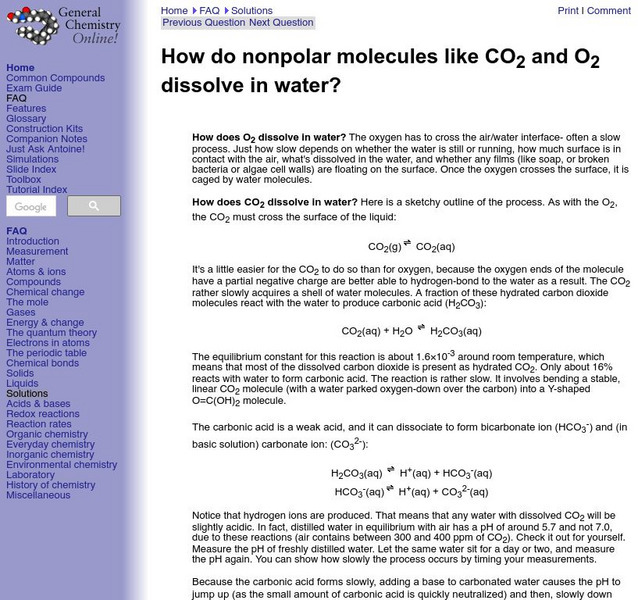
University of Frostburg: How Nonpolar Molecules Dissolve
This site from the University of Frostburg provides an explanation of the process by which nonpolar molecules dissolve in water.
Frostburg State University
Frostburg State University: Polar Solutes
Graphic representing polar solute particles, with brief accompanying notes. Part of a slide show on chemical change.
Libre Text
Libre Texts: Chemistry: Solubility and Factors Affecting Solubility
Understand how temperature, pressure, and the presence of other solutes affect the solubility of solutes in solvents.
Other
Science House: Ice Cream
Experiment shows students how to use the lowered freezing point of water to chill another mixture (ice cream) to the solid state. Teacher's notes provide background information.
San Diego State University
San Diego State University: Polar Solute
A knowledge map with clickable links related to polar solutes.
San Diego State University
San Diego State University: Nonpolar Solute
A knowledge map with clickable links related to nonpolar solutes.