Teach Engineering
Wizardry and Chemistry
No need to go to Ollivanders to buy a magic wand. In the chemistry lab activity, young magicians mix chemicals to create combustible compounds. By applying these compounds to an iron wire, they create magic wands.
Virginia Department of Education
The Rate of a Chemical Reaction
If your pupils think a catalyst is a list of their cats, then this might be the lesson for you! Young chemists study the effect of temperature, catalysts, concentration, and particle size on reaction rates during four different...
Curated OER
2000 U.S. National Chemistry Olympiad National Exam - Part I
The National Chemistry Olympiad exams are comprehensive tests covering an entire year of chemistry concepts. You can use them as practice for competing in the challenge, or simply as a review, or as an actual final exam for your...
Curated OER
Identifying and Explaining Reactions
Introduce high schoolers to chemical reactions with this series of activities. In a little over an hour, scientists observe four gas-producing reactions: the combination of hydrochloric acid and sodium hydroxide, placing pasta in...
Curated OER
2007 U.S. National Chemistry Olympiad Part II
Eight multi-step chemistry problems, including analyzing a titration, writing equations, predicting products and limiting reagents, calculating concentrations of ions, and using stoichiometry to solve for unknowns in reactions make up...
Curated OER
2001 U.S. National Chemistry Olympiad National Exam Part II
Only eight problems are on this competitive national chemistry exam. It required the balancing of chemical equations, solving stoichiometry questions, and more. This is part two of three of the national exam. Also available is a...
PBS
Color Code
Don't let your brain play tricks on you! Learners test brain reaction rates while it is receiving multiple stimuli. They time each other reading a set of color words written in different colors and again when they are written in black....
NASA
Christa's Lost Lesson: Effervescence
How are chemical reactions affected by gravity? Learners explore the phenomenon of effervescence as part of the Christa's Lost Lessons series. They compare findings in an experiment on effervescence to a video of a similar experiment in...
Utah Education Network (UEN)
Utah Open Textbook: Chemistry
Technology can help save money and add convenience. The resource offers a free textbook for a complete Chemistry course. The text begins with a review of the scientific method and continues to explain topics such as chemical bonding,...
College Board
2016 AP® Chemistry Free-Response Questions
The College Board published the mean score on the 2016 AP Chemistry exam as a 2.69, but a minimum of a three is required to earn college credit. Help pupils study for the upcoming exam with actual test questions, sample answers, and...
Kenan Fellows
Qualitative Kinetics: Examining the Effect of an Enzyme on a Reaction
Scholars learn about kinetics and buffers as they use qualitative and quantitative methods to understand enzyme rates and buffer capacity. The application of Beer's Law and spectrophotometry solidifies pupils' knowledge in the first of...
Royal Society of Chemistry
A Solid-Solid Reaction between Lead Nitrate and Potassium Iodide
Why is it so difficult to make two solid compounds react? Investigate the concepts of particle collisions and rate of reaction using a quick demonstration. The colorful experiment features two plain, white solids combining to form a...
Concord Consortium
Temperature and Reaction Rate
Does increasing temperature increase the rate of a chemical reaction? Junior chemists examine the effects of temperature on reaction rate using an engaging interactive. Pupils select the temperature of the reaction vessel, then observe...
Concord Consortium
What Is a Chemical Reaction?
Take your class inside a beaker for an up-close view of a chemical reaction! Junior chemists examine how chemical reactions occur using an interactive resource. The activity allows users to change the temperature and observe how it...
Concord Consortium
Catalysis
Get ready to kick things up a notch! Young scientists explore the effects of catalysts using a fun interactive. Learners start the reaction without using a catalyst, then add one over time to examine its effects on reaction rate.
Concord Consortium
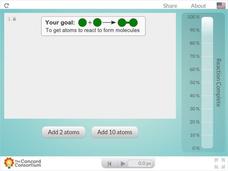
Concentration and Reaction Rate
Does concentration affect the rate of a chemical reaction? Science scholars observe and control a chemical reaction in an interactive simulation. The resource allows pupils to add atoms to the reaction vessel and monitor the reaction's...
Science Geek
Equilibrium and Le Chatelier's Principle
Time to shake up the status quo with a presentation that describes Le Chatlier's Principle and has pupils examine situations in which equilibrium is upset. Four examples show different stresses to the reaction and the resulting shift.
Herff Jones Education
Reaction Rates
Equip pupils with tools to determine reaction rates as they explore conditions that cause a reaction to increase or decrease. They also discuss why this occurs and predict the next steps as they take part in a series of...
Curated OER
Oxidation: Does Iron Burn?
Searching for a fairly easy demonstration of how oxidation triggers rust formation? The demonstration allows high school chemists to witness the rusting of metals, as large and small objects are held into a flame while triggering the...
Curated OER
Factors that Affect Reaction Rates
Collaborating chemistry pupils observe that temperatrue increases the movement of dye throughout water, stirring increases the dissolving rate of sugar cubes, and concentration of solutions increases the chemical reaction rates. These...
Curated OER
Rate and Concentration
Experimental data for a chemical reaction is available in a table. Chemistry cohorts use critical thinking skills to analyze the data and answer questions about reaction rates and reaction order. This worksheet is neatly formatted,...
Curated OER
Inquiry into Consumer Products
Students recognize different consumer products, found in and around the home, that have reactive or denaturing properties when used together. They explore chemical and physical properties of each product by identifying chemical formulas...
Curated OER
Physical and Chemical Reactions - Factors Which Affect Reaction Rate
A total of five experiments lead chemistry pros to understand the difference between physical and chemical change. They also experiment with exothermic reaction factors that affect rate of reaction. The procedures are not written in the...
Curated OER
Reaction Time 2: Zap!
Learners explore critical thinking by conducting a reaction time experiment. In this human brain lesson, students utilize a timed Internet worksheet activity to research how fast their brain works when answering questions. Learners share...