CK-12 Foundation
Ck 12: The Nuclear Model of the Atom
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will distinguish between the three main subatomic particles and understand the contributions of J. J. Thomson, Robert...
Science Education Resource Center at Carleton College
Serc: Rutherford's Enlarged: A Content Embedded Activity on Nature of Science
Through a study of Ernest Rutherford's experiments with atomic structure, students develop a deeper understanding of the nature of science. The activity is taken from a linked article by the author that was published in the Journal of...
Nobel Media AB
The Nobel Prize: Ernest Rutherford Biographical
A complete biography on the life of Ernest Rutherford, 1908 winner of the Nobel Prize for Chemistry. Details his education and his many scientific researches. Also contains a listing of his awards and achievements and has some...
Nobel Media AB
The Nobel Prize: The Nobel Prize in Chemistry 1908
At this site read about Ernest Rutherford (1871-1937 CE), the scientist who was awarded the Nobel Prize in Chemistry, "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances." This...
Houghton Mifflin Harcourt
Harcourt: Biographies: Ernest Rutherford
This is an account of Nobel Prize winner Ernest Rutherford's life. It tells of his accomplishments in the field of chemistry and physics. There are additional links to supplemental material as well.
Wikimedia
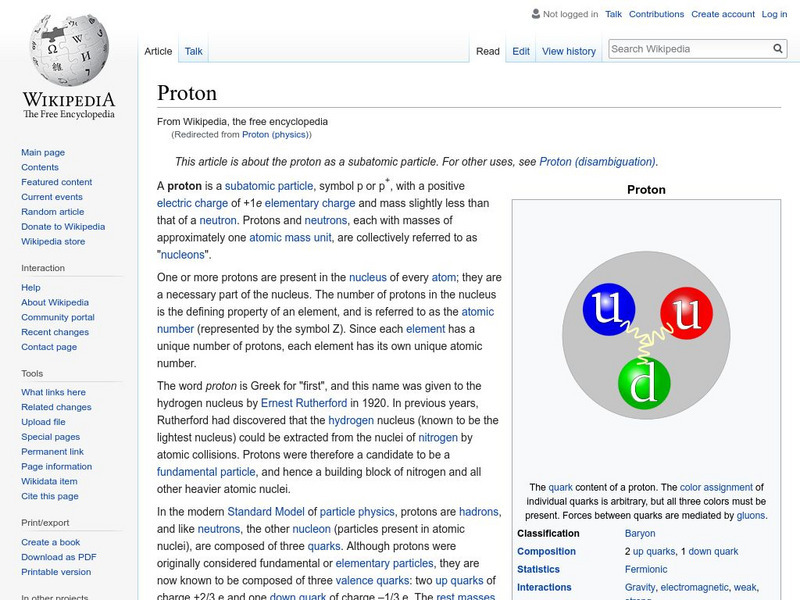
Wikipedia: Proton
Wikipedia offers information on the proton, a subatomic particle with a positive electric charge. Many hyperlinked terms.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Rutherford Scattering
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining...
Other
A World of Particles: The Neutron
A World of Particles offers a history of the discovery of the neutron, an exclusive particle in an atom.
Other
Siyavula Education: Everything Maths & Science: Models of the Atom
Discusses models of the atom developed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Includes clear illustrations and a short comprehension exercise at the end.
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
Other
Crocodile Clips: Absorb Chemistry: History of the Atom
A tutorial that presents models of the atom proposed by John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr. Each is supported by an animated illustration. Includes comprehension questions and a quiz at the end.
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
Sophia Learning
Sophia: Ernest Rutherford: Lesson 2
This lesson explains Rutherford's gold foil experiment and the discovery of the nucleus. It is 2 of 3 in the series titled "Ernest Rutherford."
National High Magnetic Field Laboratory
Magnet Academy: Geiger Counter 1908
Counting alpha particles was tedious and time-consuming work, until Hans Geiger came up with a device that did the job automatically.
National High Magnetic Field Laboratory
Magnet Academy: Timeline of Electricity and Magnetism: 1890 1899
Scientists discover and probe x-rays and radioactivity, while inventors compete to build the first radio.
National High Magnetic Field Laboratory
Magnet Academy: Timeline of Electricity and Magnetism: 1910 1929
Scientists' understanding of the structure of the atom and of its component particles grows, the phone and radio become common, and the modern television is born.
Famous Scientists
Famous Scientists: Ernest Rutherford
This short biography about Ernest Rutherford explains how he became known as the father of nuclear chemistry and physics.
CK-12 Foundation
Ck 12: Chemistry: Gold Foil Experiment: Rutherford's Atomic Model
[Free Registration/Login may be required to access all resource tools.] This site explains Rutherford's discovery of the nucleus and the planetary model of the atom and the role of gold foil experiment in refining the atomic model. It...
CK-12 Foundation
Ck 12: Chemistry: Neutrons
[Free Registration/Login may be required to access all resource tools.] Explores the discovery and properties of the neutron and the process of nuclear fission.
CK-12 Foundation
Ck 12: Chemistry Simulation: Rutherford's Gold Foil Experiment
[Free Registration/Login Required] How can we predict an atom's structure, if we cannot see an atom? Using the Rutherford's Gold Foil Experiment, make your own model and test out the model.
Concord Consortium
Concord Consortium: What Are Nature's Building Blocks?
Activity 3 of this module investigates: How do we know what's inside an atom? From Ernest Rutherford's Gold Foil Experiment to his investigation of the Plum Pudding model, students become aware of the Nuclear Model of an Atom. Also in...
Lawrence Berkeley National Laboratory
Berkeley Lab: The Atom
Presented is an overview of atomic theory concentrating on the experiments of Ernest Rutherford.
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
Michigan State University
Michigan State University: Environmental Health & Safety: Ernest Rutherford
A clear telling of Rutherford's place in the history of human understanding of radiation.