Virginia Department of Education
Physical and Chemical Properties of Water
How can you effectively provide detailed concepts of water properties to your high school class in a way they find exciting and challenging at the same time? By letting them play, of course! Through a variety of experiments, pupils...
Concord Consortium
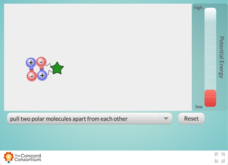
Comparing Potential Energy of a Bond
Have you reached your breaking point in looking for great resources that illustrate bond energy? Demonstrate the potential energy changes that occur when polar and non-polar bonds are broken with a stimulating simulation. Pupils pull on...
Mr. E. Science
Atoms and Bonding
I don't trust atoms because they make up everything. Budding scientists learn about famous scientists connected to atomic models, chemical, ionic, and hydrogen bonds. The presentation also presents how to count atoms in an equation,...
Curated OER
Chapter 6 - Bonds
Although there are only 16 questions here, this chemistry handout makes a terrific unit assessment. It queries youngsters on the properties of ionic and covalent compounds, relates bond length tho stability and enrgy, compares polar and...
Curated OER
Call Me Bond, Hydrogen Bond
As amazing as James Bond is, the surface tension of water does not allow him to walk on it! In this series of little lab activities, physical scientists play with the properties of water due to the hydrogen bonds and resulting polarity....
Curated OER
Bond Type
At the top of the page are a reading passage and colorful diagram that depicts the tug-of-war that occurs between bonding molecules due to electronegativity. High school chemists fill in a chart with electronegativity values, the...
Curated OER
8.4 Section Review ~ Polar Bonds and Molecules
A very neat worksheet has been produced by Pearson Education, Inc. for use in a general chemistry class. The first nine questions are fill in the blanks for a paragraph about types of bonds and electronegativity. Five true-false...
Curated OER
Chemical Context of Life & Water
Some basic chemistry concepts are fundamental to understanding biology. Learners explore how molecular structure plays a role in biological processes, especially the structure of the water molecule. The final page focuses on water's...
Curated OER
Worksheet 7 - A Practice Quiz 3
Every sort of chemical bond is touched upon during this assignment. Chemistry whizzes identify what type of bond is formed by analyzing chemical formulas or Lewis structure diagrams. Multiple choice questions also ask learners about...
University of Arizona
Ua: Chemistry Tutorial
This general tutorial begins with an explanation of the polarity of the water molecule and the effects this polarity has on the properties of water. Goes on to introduce organic molecules and has a thourough tutorial on the third page.
Ohio State University
Ohio State University: Electronegativity & Bond Polarity
Excellent graphics help this page explain the relationship between electronegativity and bond polarity.
Other
Chemguide: Electronegativity
This page explains what electronegativity is, and how and why it varies around the Periodic Table. It looks at the way that electronegativity differences affect bond type and explains what is meant by polar bonds and polar molecules.
Clackamas Community College
Clackamas Community College: Electronegativity
A good discussion of electronegativity and its effects on bond polarity and bond type.
CK-12 Foundation
Ck 12: Physical Science: Bond Polarity
[Free Registration/Login may be required to access all resource tools.] Polarity and covalent bonds.
Biology Pages
Kimball's Biology Pages: Electronegativity
This site, which is a personal site from Kimball's Biology, provides an overview with multiple examples of electronegativity.
Frostburg State University
General Chemistry: Why Oh, Nh, and Fh Bonds Are Polar
Frostburg State University provides a brief explanation to the question of why small hydrogen molecules, such as OH, NH, and FH, are very polar.
Other
University of Texas Dallas: Electron Appetites
The first section of this lecture on polarity and electronegativity discusses what polar covalent bonds are by using carbon dioxide as an example.