Concord Consortium
Concord Consortium: Molecular Workbench: Strength of Intermetallic Bonds
View two different substances made from the same two elements. Observe the different strengths of bonds between the two different substances.
Sophia Learning
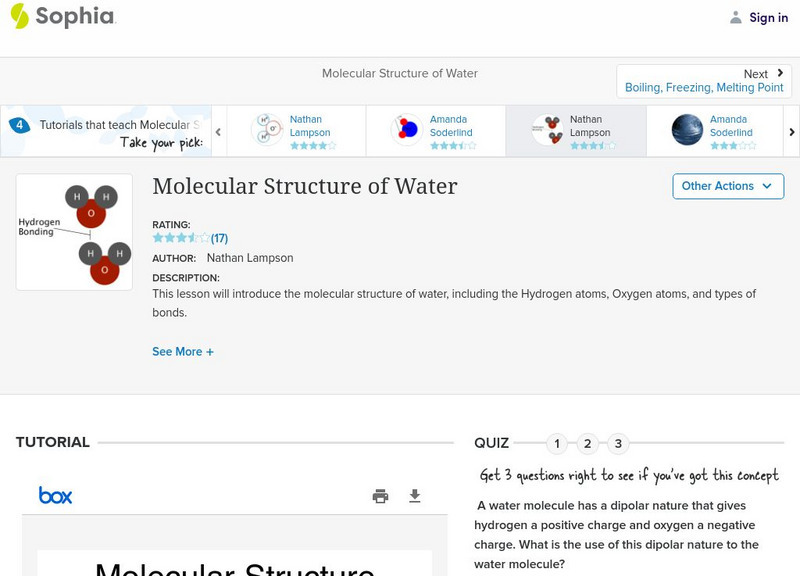
Sophia: Molecular Structure of Water: Lesson 2
This lesson will introduce the molecular structure of water, including the Hydrogen atoms, Oxygen atoms, and types of bonds. It is 2 of 4 in the series titled "Molecular Structure of Water."
Sophia Learning
Sophia: Molecular Structure of Water: Lesson 3
This lesson will introduce the molecular structure of water, including the Hydrogen atoms, Oxygen atoms, and types of bonds. It is 3 of 4 in the series titled "Molecular Structure of Water."
Educaplus (Jesús Peñas Cano)
Educaplus: Polaridad Y Diferencias De Electronegatividad [In Spanish]
Test your knowledge of Chemistry. Complete the diagram by dragging the labels to the correct location.
ClassFlow
Class Flow: Intro to Atoms
[Free Registration/Login Required] This flipchart was converted from Power Point and is used to introduce the history and concept of the Atom.
ClassFlow
Class Flow: Ionic & Covalent Compounds
[Free Registration/Login Required] This flipchart presents rules for writing and naming ionic and covalent compounds. There is a short assessment using Activotes.
Oklahoma State University
Oklahoma State University: Bonding Rules of Thumb
Simple rules to help students identify types of chemical bonding.
McMaster University
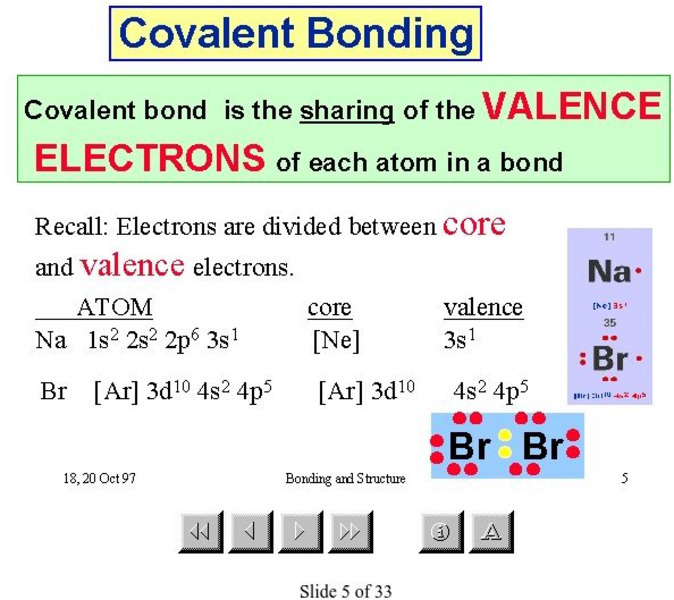
Mc Master University: Covalent Bonding
Slides 5 through 8 in this presentation from the McMaster University explain covalent bonding.
Other
University of Texas Dallas: Electron Appetites
The first section of this lecture on polarity and electronegativity discusses what polar covalent bonds are by using carbon dioxide as an example.
Curated OER
4 Different Modes of Representation of Covalent Bonds
A tutorial on covalent bonds and intermolecular forces. A good summary weak forces, including Van der Waals forces and an excellent illustration showing the relative strengths of different forces and bonds.




![Educaplus: Polaridad Y Diferencias De Electronegatividad [In Spanish] Activity Educaplus: Polaridad Y Diferencias De Electronegatividad [In Spanish] Activity](https://content.lessonplanet.com/knovation/original/180052-bebdac3e107b5cf58c094c0fe5c0176c.jpg?1661810230)