Sophia Learning
Sophia: Democritus
A guided lesson explaining Democritus' description of matter as discontinuous.
Sophia Learning
Sophia: Development of the Early Atomic Theory
This guided lesson summarizes the historical development of atomic theory.
Other
Particle Adventure Dutch Version
Dutch version of the well-known "Particle Adventure" physics website that teaches students about atoms, mass, particle physics, and quantum physics. The site discusses theories related to physics and provides other links related to the...
Thomas Jefferson National Accelerator Facility
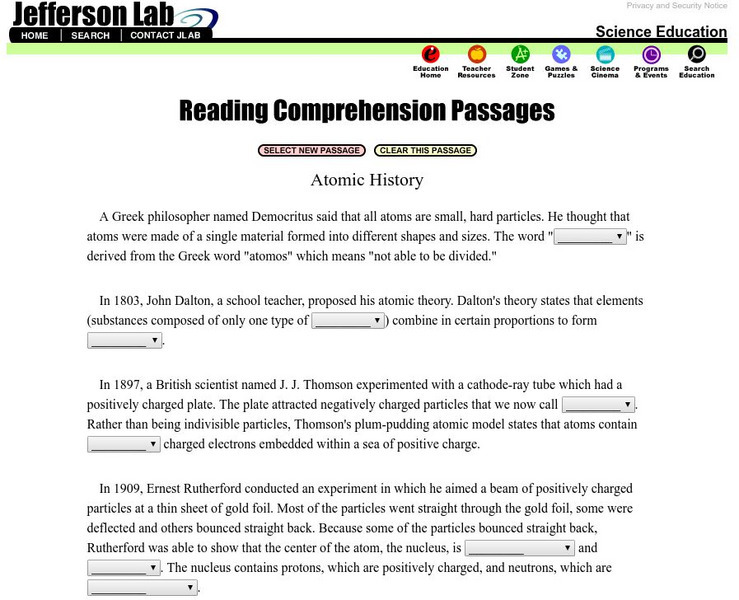
Jefferson Lab: Reading Passages: Atomic History
Read and fill in the blanks of this passage explaining atomic history. Each blank has a dropdown menu with choices. When you finish, click CHECK MY ANSWERS. If you pick a wrong answer, the right answer will be displayed along with your...
BBC
Bbc: Gcse Bitesize: Atomic Structure
This lesson focuses on the structure of atoms. All substances are made from atoms. Each atom is made of a nucleus - containing protons and neutrons - surrounded by electrons. It provides a link to an assessment.
Frostburg State University
General Chemistry Online: History of Chemistry
A compilation of frequently asked questions in the world of chemistry. Look for answers about atomic theory, periodic table, and Lavoisier in this FAQ.
Frostburg State University
General Chemistry Online: Atoms, Elements, and Ions Faq
Get the skinny on all things frequently asked about isotopes, subatomic particles and atomic theory. Find out what a "dalton" is or the difference between Na+ and Na.
University of St. Andrews (UK)
University of St. Andrews: Max Planck
This site is a mixture of a short biography and quotes on German physicist Max Planck.
CK-12 Foundation
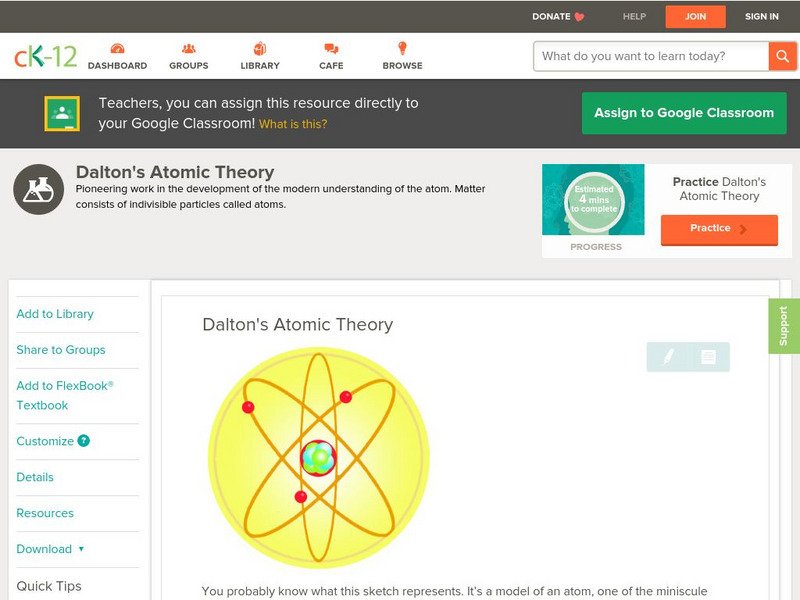
Ck 12: Physical Science: Dalton's Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Reintroduction of the idea of the atom and the billiard ball model.
Other
Chemical Heritage Foundation: John Dalton
This interesting site has a biography of John Dalton as well as other influential scientists in the field of chemistry.
Other
Dalton's Atomic Theory
This site contains Dalton's atomic theory and a brief biography about the scientist.
CK-12 Foundation
Ck 12: Chemistry Simulation: Rutherford's Gold Foil Experiment
[Free Registration/Login Required] How can we predict an atom's structure, if we cannot see an atom? Using the Rutherford's Gold Foil Experiment, make your own model and test out the model.
Frostburg State University
General Chemistry Online: Dalton's Atomic Theory
Discusses John Dalton's development of the atomic theory and the four main points of his theory. Includes references and a link to a quiz on Dalton's Atomic theory.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
How Bohr's model of hydrogen explains atomic emission spectra.
Ohio State University
Osu History Teaching Institute: The Development of Atomic Theory
Students learn about the development of atomic theory.
Lawrence Berkeley National Laboratory
Berkeley Lab: The Atom
Presented is an overview of atomic theory concentrating on the experiments of Ernest Rutherford.
National Museum of Science and Industry (UK)
Ingenious: Atomic and Nuclear Physics Images
This series of images is a combined collection of over three museums' collections of atomic and nuclear physics images. The images are sorted by pages with about nine images per page and three pages.
Simon Fraser University
Chem1 Virtual Textbook: What Is an Orbital?
Acting as part of an overview on quantum theory, this section of the site deals with electrons and orbitals. In addition to explain what an orbital is, the site explains the movement of the electron in relation to the nucleus.
Upper Canada District School Board
Tom Stretton's Chemistry Pages: Atomic Theory Early Ideas
This online slide show helps students understand the early historical thinking that contributed to modern atomic theory.
Upper Canada District School Board
Tom Stretton's Chemistry Pages: Atomic Theory Modern Ideas
This online slide show helps students understand modern scientific thinking which has shaped modern atomic theory.
PBS
Pbs: People and Discoveries
At this PBS site there is a biography of the Austrian physicist Erwin Schrodinger who lived from 1887 until 1961.
Other
Sir Joseph John Thomson
Have you ever wondered who discovered the electron? The answer is Nobel Prize winning physicist Sir Joseph John Thomson.
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
Sophia Learning
Sophia: James Chadwick
This lesson explains the discovery of the neutron and Chadwick's experimental process.