Rensselaer Polytechnic Institute
Molecules to the Max!—Educators Resource Guide
From molecules to nanotubes, an engaging unit explores the world of tiny science. Fifteen hands-on experiments and lessons engage young scientists as they learn chemistry. Discussions, worksheets, and data analysis reinforce the concepts...
Cornell University
Alka-Seltzer Rockets
Blast off! An engaging hands-on activity has pupils create rockets powered by Alka-Seltzer. They learn about the physics behind these rockets throughout the process.
Cornell University
What Is Rust?
Why do metals rust differently? Scholars experiment with metal combinations in a hands-on activity. They create unique environments with different metals and compare the rate and amount of rust for each.
Cornell University
Unknown Powders
Create a little scientific magic within your classroom! Learners mix powders and liquids and identify chemical reactions. Based on the reactions, individuals determine the identity of various powders.
Concord Consortium
Chain Reaction Between Hydrogen and Oxygen
Looking for a simple way to teach conservation of energy in chemical reactions? Pupils can observe energy changes as water forms during a chain reaction between oxygen and hydrogen using an interactive. The resource instructs users to...
Concord Consortium
Reaction Between Hydrogen and Oxygen Molecules
When molecules of hydrogen and oxygen are combined, how does water form? Science scholars observe changes in kinetic and potential energy during a chemical reaction in an interactive. The resource features easy controls that allow users...
Concord Consortium
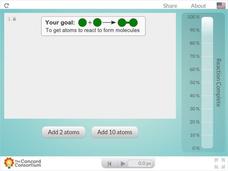
Stoichiometry and Balancing Equations
Is your stoichiometry lesson plan just not adding up? Incorporate an exciting interactive to balance things out! Chemistry scholars manipulate the number of molecules added to the reaction vessel, then observe as bonds form and break as...
Concord Consortium
Temperature and Reaction Rate
Does increasing temperature increase the rate of a chemical reaction? Junior chemists examine the effects of temperature on reaction rate using an engaging interactive. Pupils select the temperature of the reaction vessel, then observe...
Concord Consortium
What Is a Chemical Reaction?
Take your class inside a beaker for an up-close view of a chemical reaction! Junior chemists examine how chemical reactions occur using an interactive resource. The activity allows users to change the temperature and observe how it...
Concord Consortium
Making and Breaking Bonds: The Effect of Temperature
Time to turn the heat up on your next bonding lesson! Young chemists explore temperature, kinetic energy, and bonding through an interesting interactive. The controls allow individuals to vary the temperature, as well as pause progress...
Concord Consortium
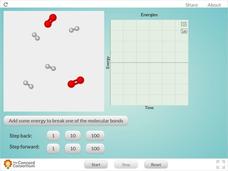
Reaction Between Hydrogen and Oxygen Atoms
Is this resource a great way for your class to observe bonding between oxygen and hydrogen? OH yeah! Scholars learn about the changes in kinetic and potential energy as molecules of oxygen and hydrogen interact. Kinetic, potential, and...
Concord Consortium
Catalysis
Get ready to kick things up a notch! Young scientists explore the effects of catalysts using a fun interactive. Learners start the reaction without using a catalyst, then add one over time to examine its effects on reaction rate.
Concord Consortium
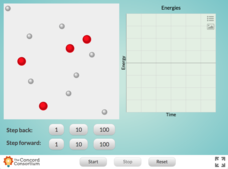
Concentration and Reaction Rate
Does concentration affect the rate of a chemical reaction? Science scholars observe and control a chemical reaction in an interactive simulation. The resource allows pupils to add atoms to the reaction vessel and monitor the reaction's...
Cornell University
Predicting Chemical Reactions
Prove the Law of Conservation of Mass through a lab investigation. A well-designed lesson asks groups to combine materials and monitor indicators for chemical reactions. Measuring the mass of the reactants and products allows individuals...
Cornell University
Chemical Reactions
Investigate the Law of Conservation of Mass through a lab exploration. Individuals combine materials to initiate chemical reactions. They monitor for signs of reactions and measure the masses before and after the reactions for comparison.
American Chemical Society
Exothermic, Endothermic, and Chemical Change
Scientists can't observe bonds breaking or forming, so how do they distinguish between exothermic and endothermic reactions? Young scholars complete two experiments to do just that. They monitor temperature change and calculate the...
LABScI
Stoichiometry: Baking Soda and Vinegar Reactions
Examine the concept of stoichiometry using common household products. Scholars perform chemical reactions and measure the reactants and products. They compare their measurements to predictions made from the chemical equations.
Chymist
Esters: An Introduction to Organic Chemistry Reactions
Scratch and sniff an introduction to organic chemical reactions. A creative lesson has individuals study the esters commonly used in scratch-and-sniff stickers and advertisements. Following the lab procedure, scholars create the organic...
Santa Monica College
Mole Ratios and Reaction Stoichiometry
Stoichiometry sounds complicated, but it really means the study of the amount of substances involved in a reaction. The sixth lesson plan in an 11-part series has scholars use stoichiometry to find the theoretical yield of a reaction....
CK-12 Foundation
Balancing Equations
Make the microscopic world of chemical reactions come to life. An engaging video demonstrates a methane-oxygen reaction. Learners see the reaction take place and observe the chemical equation being balanced.
CK-12 Foundation
Crash
Explore the chemistry behind the airbags that keep you safe in a collision. Using a simulation, your classes find the best gas to use to inflate an airbag. The simulation shows the time it takes to inflate to a maximum volume....
Santa Monica College
Single and Double Displacement Reactions
If you aren't part of the solution, you are part of the precipitate! Young chemists learn about single and double displacement reactions including precipitation reactions, neutralization reactions, and gas forming reactions. They perform...
Santa Monica College
The Composition of Potassium Chlorate
The third lesson in a series of 11 begins by using thermal decomposition of potassium chlorate to determine the mass percent of oxygen. Then a second activity allows scholars to demonstrate that the resulting residue is from a...
National Institute of Open Schooling
Adsorption and Catalysis
Adsorption, not absorption, is when atoms stick to the surface of an object, like water sticking to a grain of sand. An informative lesson delves into adsorption, teaching physical and chemisorption and the factors that affect them....