Curated OER
The Effect of Concentration Changes on Equilibrium
In this concentration changes and equilibrium worksheet, students experiment with iron (III) ions and thiocyanate ions to observe the effects of concentration on the equilibrium of solutions. Students make observations of their mixtures,...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Concentration
Learn how concentration, evaporation, and saturation affect the concentration of a solution.
CK-12 Foundation
Ck 12: Solution Concentration
[Free Registration/Login may be required to access all resource tools.] Students will calculate the concentration of solutions in units of molarity, and use molarity to calculate the dilutions of solutions.
CK-12 Foundation
Ck 12: Solubility Equilibrium
[Free Registration/Login may be required to access all resource tools.] Students write the solubility product constant expressions for nearly insoluble ionic compounds and perform calculations for compounds and solubility.
University of Colorado
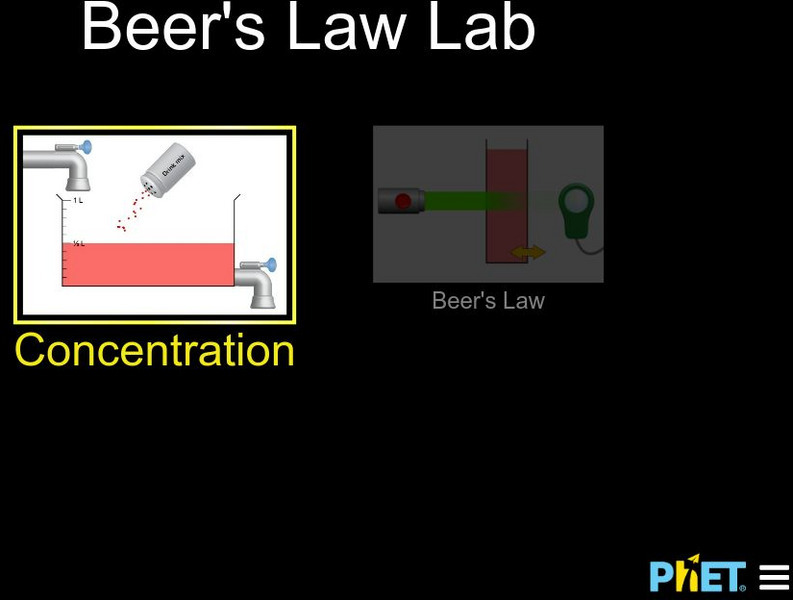
University of Colorado: Ph Et Interactive Simulations: Beer's Law Lab
"The thicker the glass, the darker the brew, the less the light that passes through." Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer!
University of Colorado
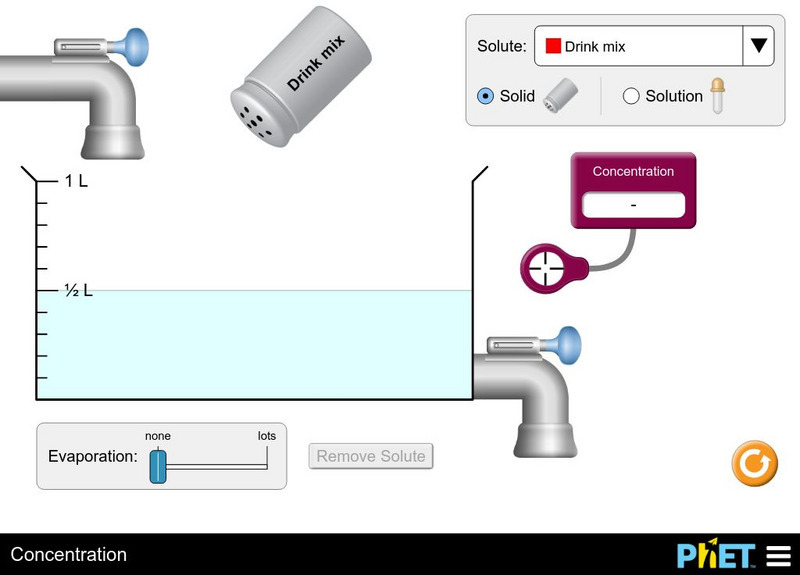
University of Colorado: Ph Et Interactive Simulations: Concentration Simulation
Students will explore changing the concentration of solutions by adding different solutes and adjusting the amount of water.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Concentration
Watch your solution change color as you mix chemicals with water. Then check molarity with the concentration meter. What are all the ways you can change the concentration of your solution? Switch solutes to compare different chemicals...
Georgia Department of Education
Ga Virtual Learning: Analysis of Organic Materials Analysis of Organic Materialsv
This comprehensive interactive tutorial continues to explore the forensic science field. Learn how organic materials of evidence are analyzed and what the various types of chromatography are, especially why they are so important to this...
Khan Academy
Khan Academy: Spectroscopy: Interaction of Light and Matter
Tutorial provides a discussion of UV-Vis spectroscopy, infrared (IR) spectroscopy, and the Beer-Lambert law.
TeachEngineering
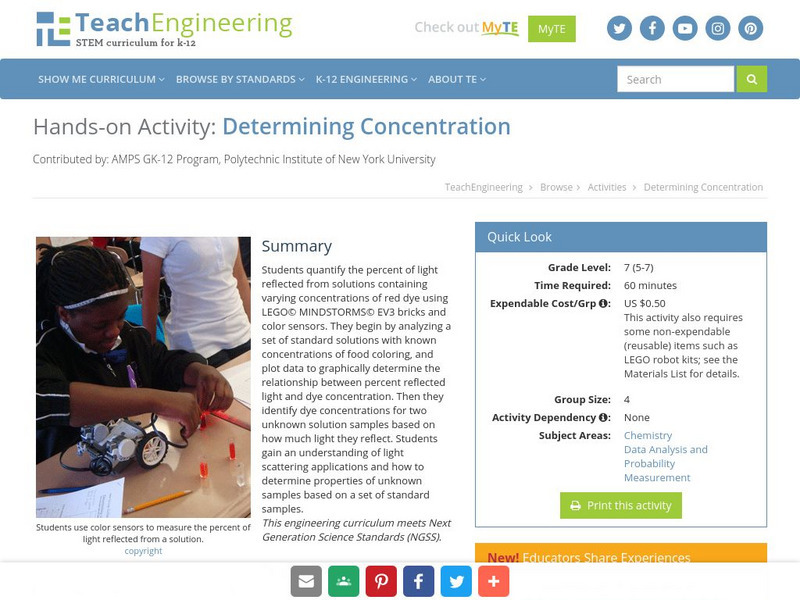
Teach Engineering: Determining Concentration
Students quantify the percent of light reflected from solutions containing varying concentrations of red dye using LEGO MINDSTORMS NXT bricks and light sensors. They begin by analyzing a set of standard solutions with known...
TeachEngineering
Teach Engineering: Concentrate This! Sugar or Salt
Students investigate the property dependence between concentrations and boiling point. First, they investigate the boiling point of various liquid solutions. Then they analyze data collected from the entire class to generate two boiling...
TeachEngineering
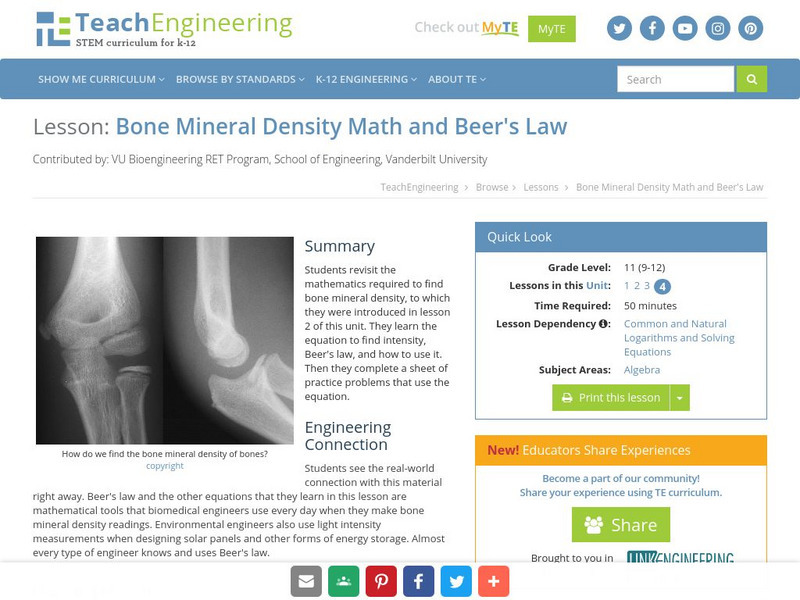
Teach Engineering: Bone Mineral Density Math and Beer's Law
In this lesson students revisit the mathematics required to find bone mineral density, to which they were introduced in Lesson 2. They will learn the equation to find intensity and how to use it. There is a sheet of practice problems...
Chemistry Collective
Chem Collective: Creating a Stock Solution
In this activity, students use the virtual lab to create dilute solutions from a concentrated stock solution of acids or bases. They must first calculate the correct volumes of concentrated acid solution and water to mix together to...
Chemistry Collective
Chem Collective: Determining the Concentration of an Unknown Solution
Using the Virtual Laboratory design, students perform an experiment to determine the concentration of the unknown HCl solution to four significant figures.
Chemistry Collective
Chem Collective: Determining Reagent Concentrations
Using the Virtual Laboratory, design an experiment to quickly determine which of the two reagents, HCl or NaOH, is 10 times more concentrated than the other.
Chemistry Collective
Chem Collective: Determine the Concentration of Acetic Acid in Vinegar
A virtual lab where students determine the concentration of acetic acid in vinegar using a 0.110 M NaOH standard solution and an acid-base indicator, phenolphthalein.
Chemistry Collective
Chem Collective: Unknown Concentration of Dna Solution Problem
In this advanced limiting reagent problem, students use the virtual lab to determine the concentration of a solution of DNA by reacting it with known amounts of a fluorescent dye which binds to the DNA.
Chemistry Collective
Chem Collective: Unknown Silver Chloride
Determine the concentration of silver ion in a Silver Nitrate solution using gravimetric analysis.
Texas Instruments
Texas Instruments: Determine Concentration of a Solution: Beer's Law
Students can use a Colorimeter to determine the relationship between concentration and absorbance of nickel sulfate solution (Beer's law). They will then determine the concentration of a unknown sample using the standard curve.
Texas Instruments
Texas Instruments: Phosphate Analysis
One method of measuring phosphate concentration is by using a Colorimeter. This instrument measures the amount of light transmitted through a sample at a given wavelength.The amount of light absorbed is mathematically related to the...
Crescent Public Schools
The Internet Science Room: Solution Concentration
Students have the opportunity to study examples and worked out example problems to further their understanding of chemical solution concentration.
TED Talks
Ted: Ted Ed: Making Waves: The Power of Concentration Gradients
The constant motion of our oceans represents a vast and complicated system involving many different drivers. Sasha Wright explains the physics behind one of those drivers- the concentration gradient- and illustrates how our oceans are...
BBC
Bbc: Gcse Bitesize: Chemical Calculations
You should be able to calculate the masses of reactants and products from balanced equations, and the formula of a compound from information about reacting masses.
Sophia Learning
Sophia: Definition of Concentration
A narrated screencast which defines concentration, and illustrates some examples of different concentrations of solutions. [1:42]