American Association of Physics Teachers
Com Padre Digital Library: Open Source Physics: Circular Well Model
This downloadable java simulation models the 2D energy eigenstates of a particle trapped in a very deep two-dimensional circular well.
Vision Learning
Visionlearning: Atomic Theory and Structure: Quantum Numbers and Orbitals
Explanation of advanced atomic theory using The Schrodinger equation.
Wolfram Research
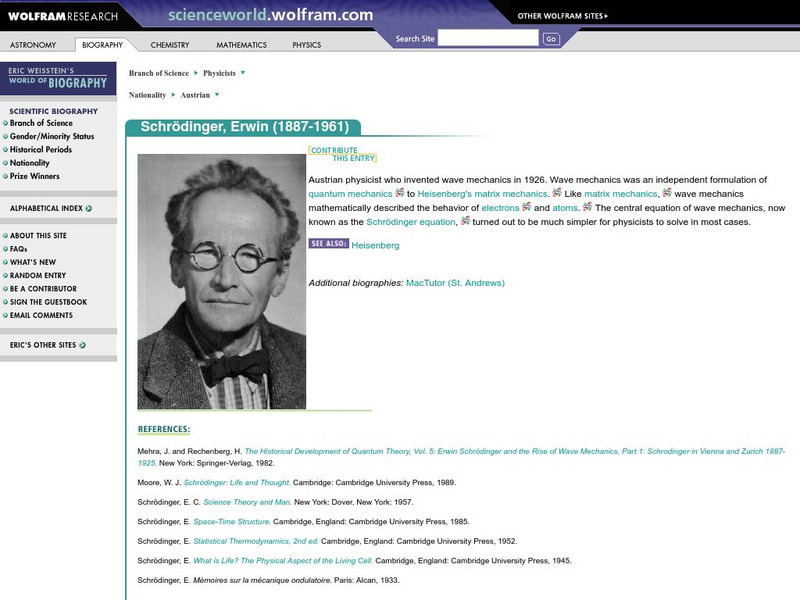
Wolfram Science World: Erwin Schrodinger
ScienceWorld.com offers a brief biography and image of Austrian physicist and inventor of wave mechanics, Erwin Schrodinger.
Dartmouth College
Dartmouth College: Chem Lab: Electrons in a Box
This site has an interactive component that allows you to change the mass, number of electrons and the length of the box and see how it affects the energy levels. Requires Java.
Libre Text
Libre Texts: Physics: Electron in a Box
What happens when an electron is trapped in a one-dimensional box? Take a look at these theories and equations applied to examples which can be illustrated this way.
Other
Chemtopics: Atomic Orbitals & Electron Configurations [Pdf]
Solutions to the Schrodinger wave equation give four types of atomic orbitals. Explanation of how quantum numbers specify these orbitals.
Science Struck
Science Struck: What Is Ionization Potential
Explains what ionization potential is and how to calculate it.
Science Struck
Science Struck: Electron Cloud Theory Explained
Explains the Uncertainty Principle, wave-particle duality, what the electron cloud model is, and how it came about as a result of the Schrodinger equation for Hydrogen.
Encyclopedia Britannica
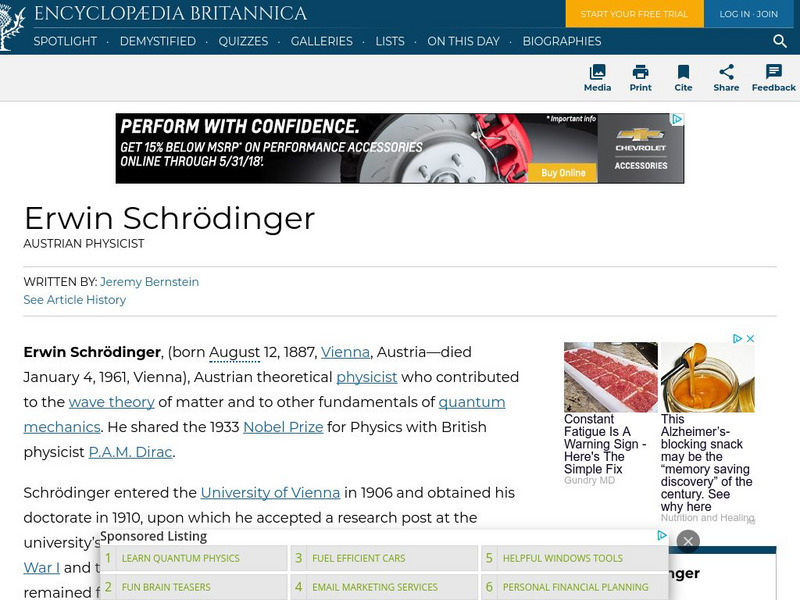
Encyclopedia Britannica: Erwin Schrodinger
This site gives brief biographical information about Austrian physicist, Erwin Schrodinger.
Other
Chemtopics: Understanding the Schrodinger Eqn.
The Schrodinger equation specifies atomic orbitals which are occupied by an electron. Quantum numbers can identify a unique energy level for each electron (set up in a PowerPoint presentation).