Shodor Education Foundation
Shodor: The Arrhenius Equation
This Shodor Educational Foundation site provides information on the Arrhenius equation and gives examples of its use.
University of Arizona
Ua: Chemistry Tutorial
This general tutorial begins with an explanation of the polarity of the water molecule and the effects this polarity has on the properties of water. Goes on to introduce organic molecules and has a thourough tutorial on the third page.
Science Museum of Minnesota
Science Museum of Minnesota: Experiment Solving Dissolving
Try this experiment to find out what happens to chalk when vinegar is added to it.
University of Nebraska
David Brooks: Henry's Law Experiment
At this site learn about Henry's law through this hands-on experiment. Lab instructions are complete including step-by-step pictures an worksheet.
Science Education Resource Center at Carleton College
Serc: Group and Periodic Properties Lab
Learners observe and perform experiments with sodium, potassium, calcium, magnesium, sulfur ,and phosphorus. The discover trends down groups, across periods, and having to do with the pH of metal oxides vs. nonmetal oxides.
Science Education Resource Center at Carleton College
Serc: Investigating Ions: Copper Topping
Young scholars will utilize a few household items to create a thin copper coating on items without using electricity.
Science is Fun
University of Wisconsin: Exploring Acids and Bases
Get a brief overview of acids, bases and pH. Explore the pH of a number of different household substances using an extract of red cabbage.
Other
Chemguide: P H Curves (Titration Curves)
On this site from Chemguide, the titration curves for various acid-base combinations (weak acid/strong base, strong acid/strong base, etc.) are talked about with examples of each given.
Other
Johnson County Community College: P H Scale
Colorful chart of the pH scale, including example chemicals.
Other
Csu Stanislaus: Titration
Check out this titration experiment tutorial. Involves titration of weak acids with strong bases and weak bases with strong acids.
Other
Fernbank: Naming Chemical Compounds
Learn how to name chemical compounds. Scientist world over use a standard method for naming chemical compounds based on an international standard that makes working with chemicals not only precise but safe as well. Includes quiz.
National Institute on Drug Abuse
National Institute on Drug Abuse: Club Drugs
This resource provides information and addresses concerns over popular "club drugs," including ecstasy. Read about the side effects of ecstasy, GHB, Ketamine, Rohypnol, Methamphetamine, and LSD.
University of Waterloo (Canada)
University of Waterloo: Strong Acids and Bases
This site from the University of Waterloo contains information on strong acids and bases. The information is fairly in-depth with good formulas and examples. A great site to check out on the subject.
University of Waterloo (Canada)
Univ. Of Waterloo: Conjugate Acid Base Pairs
This page, created by a chemistry professor from the University of Waterloo, provides an introduction to conjugate acid-base pairs.
University of Waterloo (Canada)
Univ. Of Waterloo: Conjugate Acids of Bases
This site, created by a University of Waterloo Chemistry professor, applies conjugate pair theory. It also deals with the ionization constants for acids and bases.
Clackamas Community College
Clackamas Community College: Amphoterism
Amphoterism is explained and specific examples are given at this site. Includes an explanation of the self-ionization of water and the reaction of some metal hydroxides.
Other
Organic Chemistry on Line: Acids and Bases
The bottom of this page gives an excellent idea of what Lewis acids and bases are. Inculdes graphics to aid in understanding.
TOPS Learning Systems
Tops Learning Systems: Top Science: Cabbage Chemistry [Pdf]
Experiment using water from boiling red cabbage to determine if a material is an acid, a base, or neutral.
Sophia Learning
Sophia: What Is an Acid?: Lesson 2
This lesson will explain what an acid is, what the pH of an acid is, and give an overview of the general properties of acids. It is 2 of 4 in the series titled "What is an acid?"
Sophia Learning
Sophia: What Is an Acid?: Lesson 4
This lesson will explain what an acid is, what the pH of an acid is, and give an overview of the general properties of acids. It is 4 of 4 in the series titled "What is an acid?"
Exploratorium
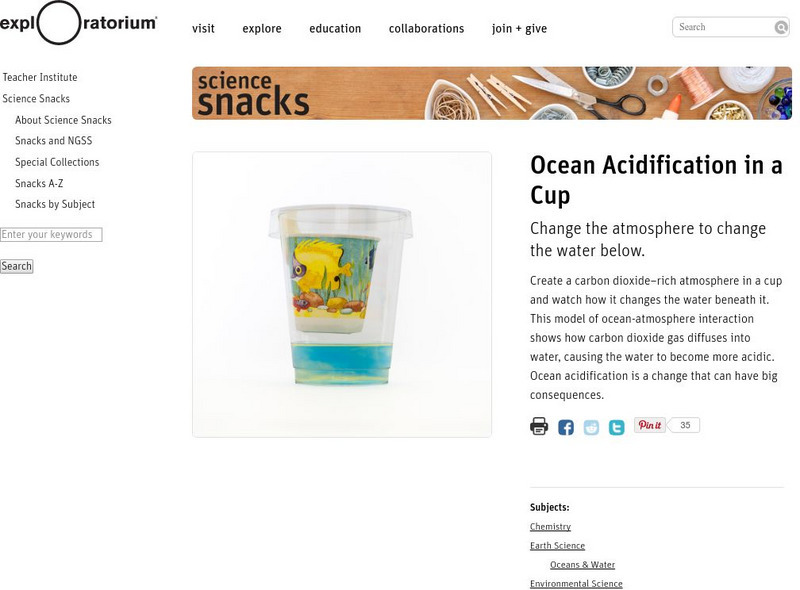
Exploratorium: Science Snacks: Ocean Acidification in a Cup
In this activity, you can observe how ocean acidification can have big consequences. This activity has students creating a cup that has a carbon dioxide-rich atmosphere that allows them to watch how the water changes beneath the atmosphere.
Khan Academy
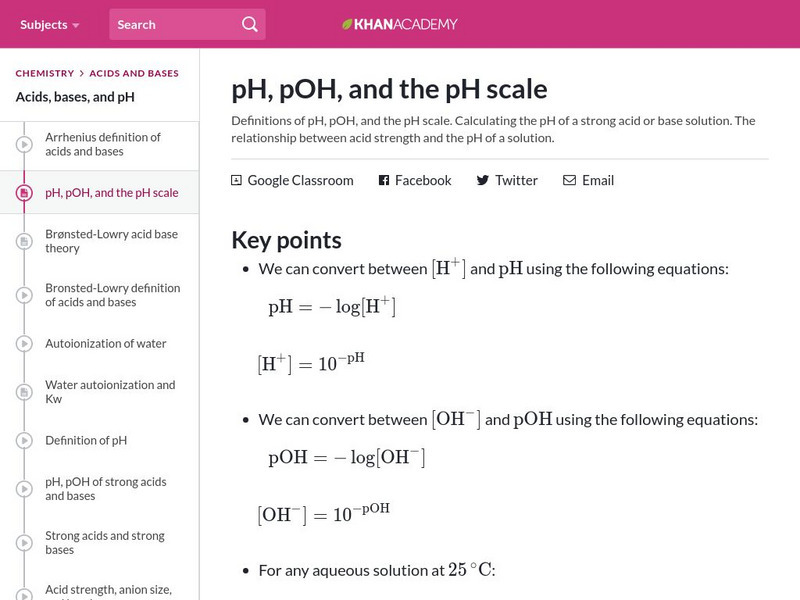
Khan Academy: P H, P Oh, and the P H Scale
Definitions of pH, pOH, and the pH scale. Practice calculating the pH of a strong acid or base solution. Learn the relationship between acid strength and the pH of a solution.
American Chemical Society
Middle School Chemistry: Neutralizing an Acidic Solution
Learn how an acidic solution is neutralized.
National Institute on Drug Abuse
Nida: Information on Common Drugs of Abuse
A comprehensive look at common drugs, including a link to each drug where you can find research reports, infofacts, publications, and notes.