Science Museum, London
Science Museum: Online Stuff: Atomic Firsts
Read about three famous British atomic physicists, each of whom won a Nobel Prize. J.J. Thompson discovered the electron, Ernest Rutherford successfully split an atom, and George Paget Thomson proved that electrons had wave-like properties.
Michael Blaber, PhD
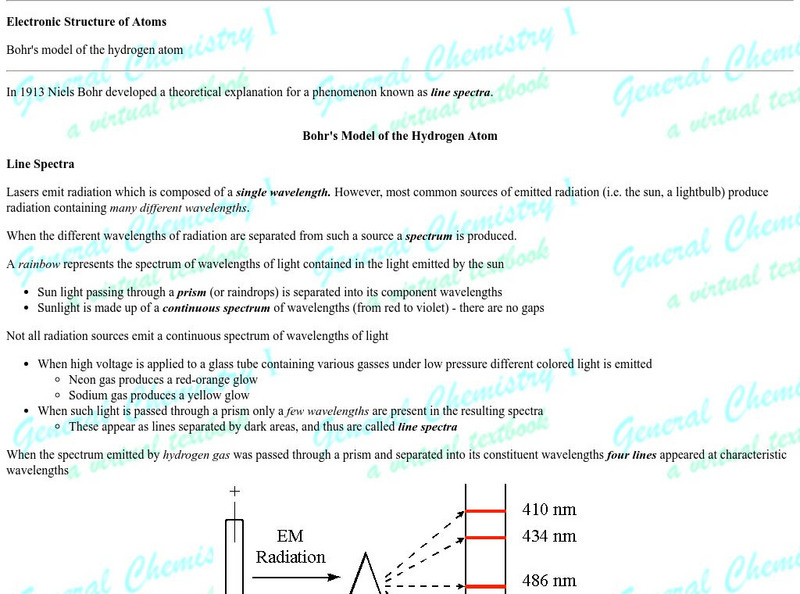
Florida State University: Electronic Structure of Atoms: Electron Configurations
Florida State University provides this article on electronic configurations of atoms and Hund's rule.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: It's Elemental Element Math Game!
Learn how to read the periodic table of elements as you solve these Math questions about the number of protons, neutrons, electrons or nucleons in an atom of an element. You can choose how many questions to answer, and how complex they...
Other
American Institute of Physics: Discovery of the Electron
Use this resource to read about the "Experiments by J.J. Thomson in 1897 [which] led to the discovery of a fundamental building block of matter." This exhibit is organized into several sections, including: "Mysterious Rays" and "1897...
CK-12 Foundation
Ck 12: Structure of the Atom
[Free Registration/Login may be required to access all resource tools.] Students learn about the important discoveries of subatomic particles, and how they led to our current understanding of the atom.
Nobel Media AB
The Nobel Prize: The Nobel Prize in Physics 1905
This Nobel E-Museum website commemorates the work of Philipp von Lenard and his Nobel prize achievement. This detailed resource includes a biography, Lenard's Nobel Lecture, and the "The Nobel Prize in Physics 1905 Presentation Speech."
Nobel Media AB
The Nobel Prize: The Nobel Prize in Physics 1923: Robert Andrews Millikan
This Nobel website on the life and scientific work of Robert A. Millikan includes a biography, images, and internet resources for further reading and research. Also included are the 1923 "Presentation Speech" which praised Millikan's...
University of St. Andrews (UK)
University of St. Andrews: Werner Heisenberg
German born Werner Heisenberg was a notable physicist who worked on numerous projects, including quantum theory.
Utah Education Network
Uen: Flowing Electrons
For this fifth grade activity, students explore electric circuits by acting out different types, then construct circuits themselves.
Nobel Media AB
The Nobel Prize: The Nobel Peace Prize 1962
Read about Linus Carl Pauling, the winner of the 1962 Nobel Peace Prize. This website is organized into the following sections: "Presentation Speech," "Biography," "Nobel Lecture," "Other Resources," and "The Nobel Chemistry Prize 1954."
Chem4kids
Chem4 Kids: Fluorine
Here you can find some great information about the 9th element in the periodic table, "fluorine." Content focuses on fluorine's electrons, where you can find fluorine in nature and in the home, and how fluorine combines with other elements.
Annenberg Foundation
Annenberg Learner: Interactives: The Periodic Table
An interactive website where students learn about the basics of an atom, periodic tables organization, and the structure and properties of matter. Module includes an introduction and five lessons that are followed by a quiz and an...
Annenberg Foundation
Annenberg Learner: Interactive Periodic Table of the Elements
A fun way to learn about the periodic table! This interactive table allows students to investigate the basic information of an element as well as explore group and family characteristics.
Simon Fraser University
Chem1 Virtual Textbook: Atomic Electron Configurations
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses a series of topics related to electrons including atoms that have only one electron, effects of...
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Other
University of Texas: Tabled Discussion
At this University of Texas site, atomic orbital occupancy, quantum numbers, the Aufbau Principle, and periodic trends are described in detail.
TeachEngineering
Teach Engineering: Mixtures and Solutions
This unit covers introductory concepts of mixtures and solutions. Students think about how mixtures and solutions, and atoms and molecules can influence new technologies developed by engineers. The first lesson explores the fundamentals...
Frostburg State University
General Chemistry Online: Atoms & Ions
This site from the General Chemistry Online of the Frostburg State University provides a review of the history of atomic theory, the discovery of the electron, and the discovery of the nucleus. Details on weighing atoms, ion charges,...
Concord Consortium
Concord Consortium: Stem Resources: Atomic Structure
Introduces learners to atomic models of the past and present, focusing on the orbital model and an explanation of its basis. Learners then have the opportunity to "make an atom" and contrast it with an ion, followed by an isotope. The...
University of St. Andrews (UK)
University of St. Andrews: Wolfgang Pauli
Check out this short look at the life of 1945 Nobel Prize winner, Wolfgang Pauli. Make sure to look at all the links at the bottom of the page too.
PBS
Pbs Learning Media: Periodic Table of the Elements
This interactive periodic table developed for Teachers' Domain provides detailed information about the chemical properties of elements and illustrates the electron configurations that determine those characteristics.
American Chemical Society
Middle School Chemistry: Represent Bonding With Lewis Dot Diagrams
Students draw and interpret Lewis dot diagrams for individual atoms and both covalent and ionic compounds.
American Chemical Society
Middle School Chemistry: Protons, Neutrons, and Electrons
Investigate why a charged object is attracted or repelled by another charged object. Explore the concept that the attraction between positive protons and negative electrons holds an atom together.
Lawrence Berkeley National Laboratory
Berkeley Lab: The Particle Adventure
Visit this site for an interactive tour of the atom and all aspects of particle physics. View the animations available with almost every description on this site. A great place for the fundamentals of particles and forces including a...