Khan Academy
Khan Academy: The Periodic Table, Electron Shells, and Orbitals
The Bohr model and atomic orbitals. Using an element's position in the periodic table to predict its properties, electron configuration, and reactivity.
Concord Consortium
Concord Consortium: Crookes Tube
Simulating J.J. Thomson's discovery of the electron. Participate in a simulation where two electrodes are connected to a high voltage source and see them produce cathode rays.
Georgia State University
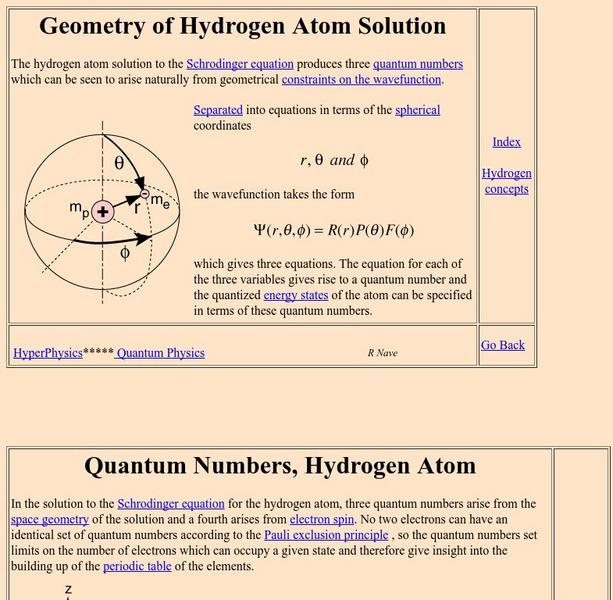
Georgia State University: Hyper Physics: Quantum Numbers, Hydrogen Atom
This tutorial contains links to explanations of the four different quantum numbers (principal, orbital, magnetic, and spin). Equations for each are provided.
University of Maryland
Um: Quantum Mechanics of One and Two Electron Atoms
An explanation of Hund's rule and how to apply it to quantum mechanics. A mathematical-based explanation.
Wolfram Research
Wolfram Science World: Sir Joseph John Thomson (1856 1940)
This site provides a brief biography of the english physicist J.J. Thomson.
Oklahoma State University
Oklahoma State University: Key Questions
A quiz on chemical bonding and valence electrons.
University of Colorado
University of Colorado: Physics 2000: The Pauli Exclusion Principle
This site from the University of Colorado at Boulder has great information on the Pauli Exclusion Principle. Pictures are provided along with links to additional information. The information provided is not in-depth and it is...
University of Colorado
University of Colorado: Physics 2000: Periodic Table: Valences and the Periodic Table
The periodic table was laid out using valences or the electrons in the outermost shell.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Spin
A basic explanation of spin and how it relates to electrons and quantum numbers.
University of Guelph
The Carbon Family: Electron Binding Energies
A very advanced site on the elements of the Carbon Family. This site describes the electron binding energies of each in a detailed graph.
Simon Fraser University
Chem1 Virtual Textbook: What Is an Orbital?
Acting as part of an overview on quantum theory, this section of the site deals with electrons and orbitals. In addition to explain what an orbital is, the site explains the movement of the electron in relation to the nucleus.
Simon Fraser University
Chem1 Virtual Textbook: Electrons
Acting as part of an overview on quantum theory, this section of the site deals with questions related to electrons, such as "If the electron cannot be localized, can it be moving?" and "Why does the electron not fall into the nucleus?"
Thomas Jefferson National Accelerator Facility
Jefferson Lab: Beta Decay
This site from Jefferson Lab provides a description of beta decay along with two helpful formula examples. Several links are provided throughout this page for additional information on related subjects.
Other
Sir Joseph John Thomson
Have you ever wondered who discovered the electron? The answer is Nobel Prize winning physicist Sir Joseph John Thomson.
Other
Wave Mechanics: Prince Louis De Broglie
A brief explanation of Louis de Broglie's original problem with the wave nature of the electron. With links to his original paper on the subject and an animated graphic of the wave.
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
Other
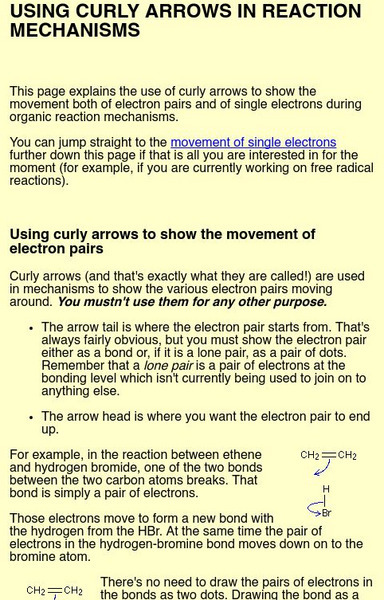
Using Curly Arrows in Reaction Mechanisms
This page explains the use of curly arrows to show the movement both of electron pairs and of single electrons during organic reaction mechanisms.
Other
Chemtopics: Atomic Orbitals & Electron Configurations [Pdf]
Solutions to the Schrodinger wave equation give four types of atomic orbitals. Explanation of how quantum numbers specify these orbitals.
University of Colorado
University of Colorado: Physics 2000: Origin of the Periodic Table: Valence Electrons
Valence electrons are defined in terms of electron configuration.
Other
Fact index.com: Balmer Series
Fact-Index.com offers a brief dictionary definition of the Balmer series, including hyperlinked terms.
Other
Fact index.com: Paschen Series
Fact-Index.com offers a dictionary definition of the Paschen series, including hyperlinked terms.
Other
American Institute of Physics: Proton Transistor Memory
Describes a new use for hydrogen ions (protons, of course). Shows that protons are not just old hat.
Quia
Quia: Mr. Snyder's Game of Atomic Terms
A short matching quiz on basic terminology related to atomic structure. Answers provided.