Other
Ap Physics Lab: Energy Levels of the Hydrogen Atom
A lab activity from an AP Physics course. Students measure the energy changes associated with electron level transitions to the second energy level for hydrogen gas. Includes directions and suggestions. Ideal for a student project or lab...
New York University
New York University: Law of Conservation of Energy
Site presents a straightforward presentation of Rutherford's work concerning the Law of Energy Conservation. Provides an adequate summary of early science on the nucleus, and includes much needed illustrations.
Sophia Learning
Sophia: Ionization Energy: Lesson 2
This lesson will define ionization energy. It is 2 of 3 in the series titled "Ionization Energy."
Sophia Learning
Sophia: Ionization Energy: Lesson 3
This lesson will define ionization energy. It is 3 of 3 in the series titled "Ionization Energy."
Sophia Learning
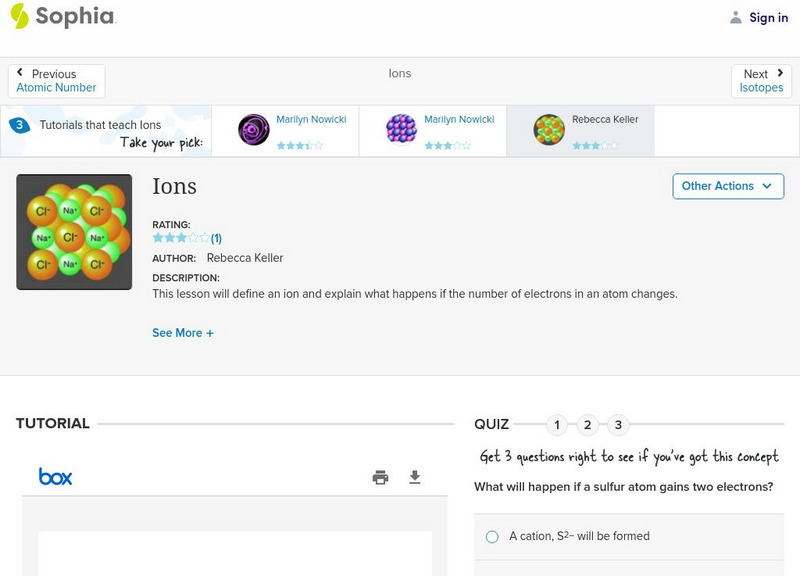
Sophia: Ions: Lesson 2
This lesson will define an ion and explain what happens if the number of electrons in an atom changes. It is 2 of 3 in the series titled "Ions."
Sophia Learning
Sophia: Jj Thomson: Lesson 1
This lesson explains JJ Thomson's experiments that led to the discovery of the electron. It is 1 of 3 in the series titled "JJ Thomson."
Sophia Learning
Sophia: London Dispersion Force Identification: Lesson 1
This lesson will explain how to identify molecules that exhibit London dispersion forces. It is 1 of 2 in the series titled "London Dispersion Force Identification."
Sophia Learning
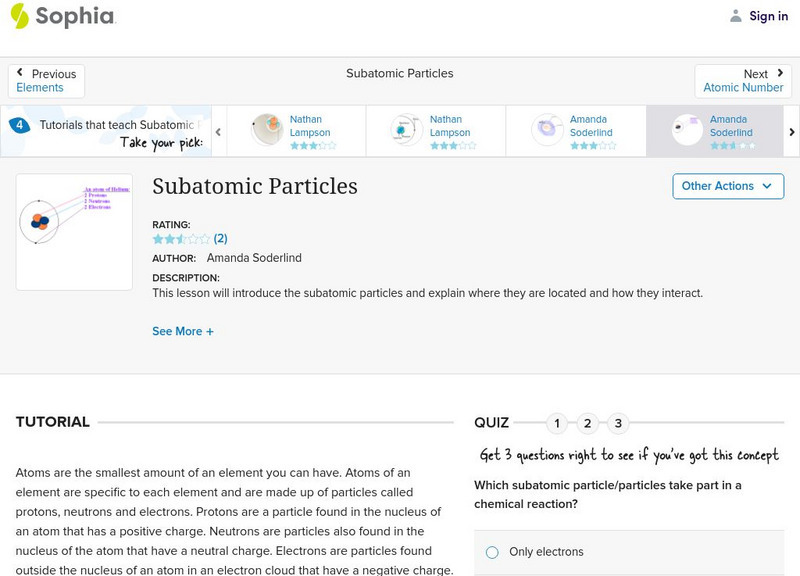
Sophia: Subatomic Particles: Lesson 3
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 3 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 4
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 4 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 6
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 6 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 7
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 7 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 5
Describe the difference between the subatomic particles, including their masses, locations, and charges. This lesson is 5 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: The Electron: Lesson 3
This lesson will explain that electrons are negatively charged particles with negligible mass and are found in pairs in orbitals surrounding the nucleus of an atom. It is 3 of 3 in the series titled "Subatomic Particles: The Electron."
Sophia Learning
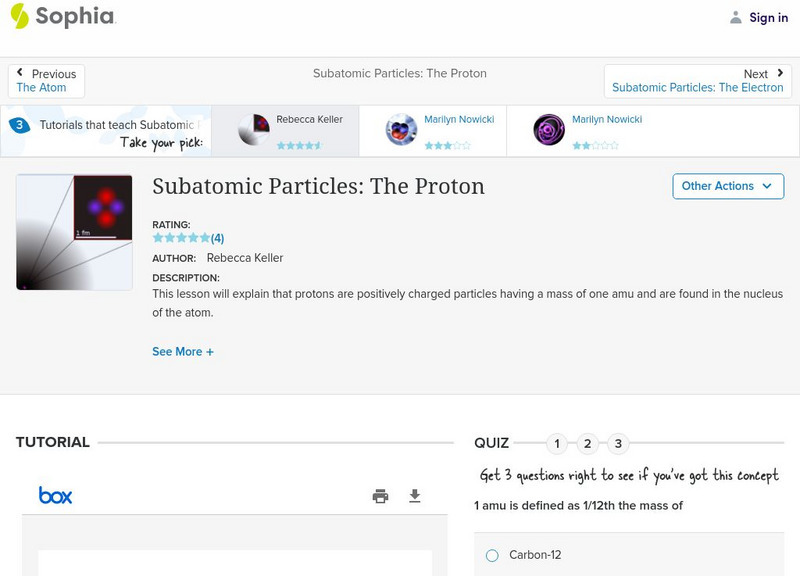
Sophia: Subatomic Particles: The Proton: Lesson 2
This lesson will explain that protons are positively charged particles having a mass of one amu and are found in the nucleus of the atom. It is 2 of 3 in the series titled "Subatomic Particles: The Proton."
Sophia Learning
Sophia: The Atom: Lesson 2
This lesson will illustrate that an atom is mostly empty space and has a positively charged, massive core (containing both protons and neutrons called the nucleus) surrounded by negatively charged electrons. It is 2 of 3 in the series...
Sophia Learning
Sophia: Atoms: Lesson 3
This lesson will provide an understanding of the basic chemistry of atoms. It is 3 of 5 in the series titled "Atoms."
Sophia Learning
Sophia: Atoms: Lesson 4
This lesson will provide an understanding of the basic chemistry of atoms. It is 4 of 5 in the series titled "Atoms."
Sophia Learning
Sophia: Atoms: Lesson 5
This lesson will provide an understanding of the basic chemistry of atoms. It is 5 of 5 in the series titled "Atoms."
Sophia Learning
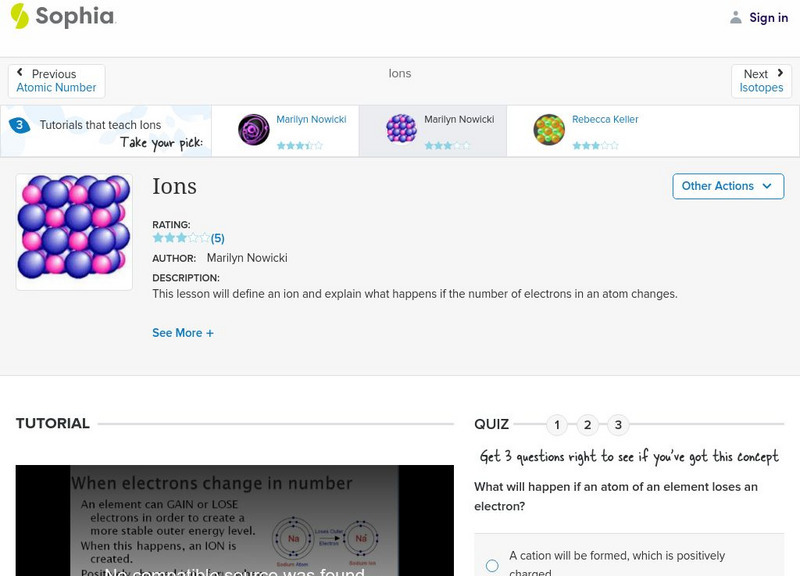
Sophia: Ions: Lesson 1
This lesson will define an ion and explain what happens if the number of electrons in an atom changes. It is 1 of 3 in the series titled "Ions."
Sophia Learning
Sophia: Subatomic Particles: The Neutron: Lesson 2
This lesson will explain that neutrons are particles in the nucleus that have no charge and a mass of one amu. It is 2 of 3 in the series titled "Subatomic Particles: The Neutron."
Physics Classroom
The Physics Classroom: Electrophorus by Induction
This tutorial offers an animation depicting the induction charging of an electrophorus plate by the process of induction. Animation depicts the movement of electrons between plat and ground. The animation is accompanied by explanations...
Stanford University
Stanford Encyclopedia: j.j. Thomson and the Electron
This site from the Stanford Encyclopedia of Philosophy offers a historical review of the discovery of the electron by J. J. Thomson and how he came to his conclusions performing experiments witha cathode ray tube.
ClassFlow
Class Flow: Atomic Structure: Parts of the Atom
[Free Registration/Login Required] This flipchart defines the parts of the atom. It uses Activotes for assessment.
Simon Fraser University
Chem1 Virtual Textbook: Movement of the Electron
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site seeks to answer the question, "Why doesn't the electron fall into the nucleus?"