Curated OER
The Photoelectric Effect
A single-page learning exercise describes the photoelectric effect of metals exposed to light. The kinetic energy equation is given, and physicists learn how to use it for problem solving. Compact in content, this can be used as a...
Curated OER
Spectroscope
Young scholars examine how to apply conservation of energy and properties of matter to a spectroscope. In this energy lesson plan students build their own spectroscope and observe three kinds of spectra.
Curated OER
Kyoto Protocol
In this environment worksheet, students read an article about setting up the Kyoto Protocol, an international treaty. They identify the meaning of human environment and what the Earth's greenhouse effect is. Students also explain the...
Curated OER
CO2: How Much Do You Spew?
Learners analyze the energy consumption of a household to see the amount of carbon dioxide they add to the atmosphere each year. In this energy consumption lesson students calculate carbon emissions and discuss sources of carbon dioxide.
Curated OER
You Do It and You Clean It Up
Students explore pollution. In this pollution lesson students participate in activities to help them better understand industrial pollution.
Curated OER
Honors Chemistry I
In this honor chemistry I worksheet, students use all available resources to answer each question given. Students apply their knowledge of light, quantum theory of light, Bohr's model, photoelectric theory.
Curated OER
Thermodynamics Homework Problem Set
In this chemistry instructional activity, students determine the equilibrium pressure of NO at each temperature listed. Then they explain why lowering the combustion temperature would have any effect on the NO emission. Students also use...
University of Colorado
University of Colorado: Physics 2000: Spectral Lines
Several pages from an excellent site which describe the science of spectroscopy. The unique atomic emission (and absorption) line spectrum of elements are illustrated and explained. Includes a Java applet depicting the quantum energy...
PBS
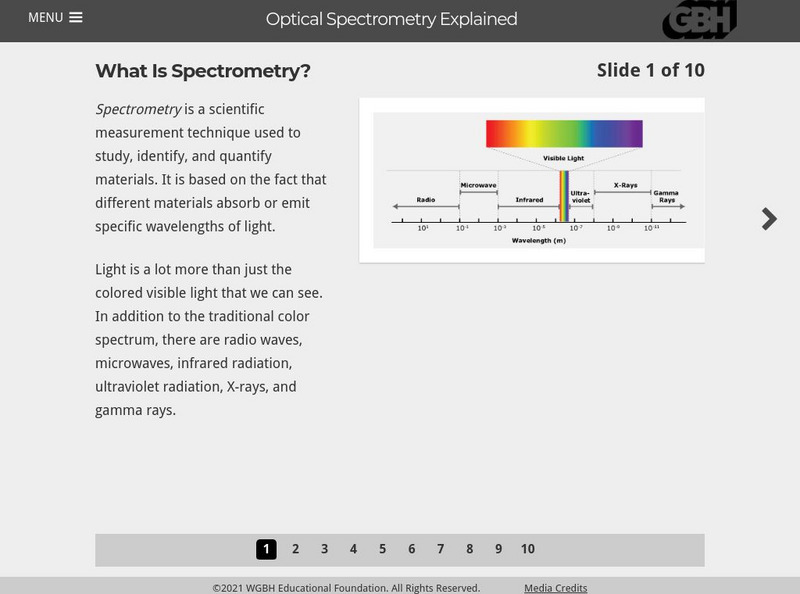
Pbs Learning Media: Spectrometry Explained
This interactive tutorial illustrates how spectrometry is used to study, identify, and quantify materials.
Georgia State University
Georgia State University: Hyper Physics: Hydrogen Energies and Spectrum
This site from Georgia State University gives information on the transitions of electrons between energy levels. The energy levels for electrons in the hydrogen atom are discussed. The Rydberg equation is stated and electron transitions...
Friesian School
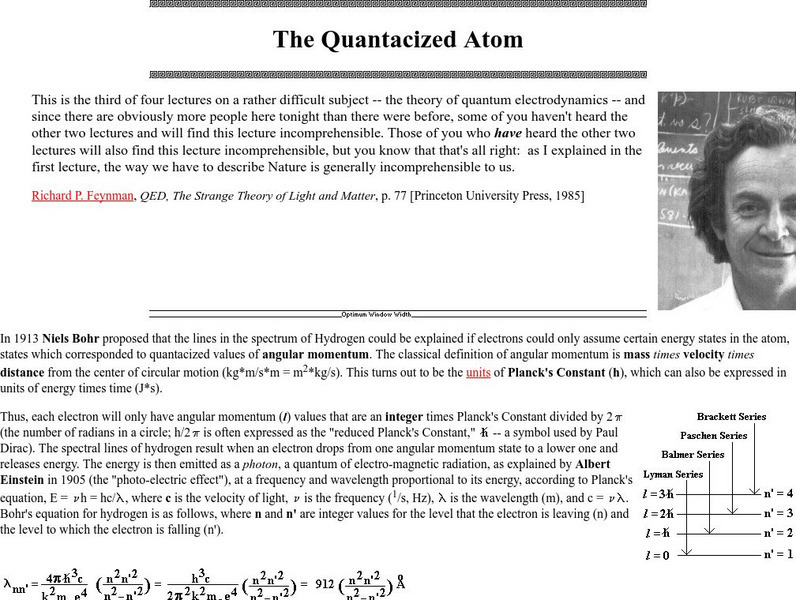
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Other
U.s. Environmental Protection Agency: Smog City 2
An interactive air pollution simulator that allows students to control the smog in an imaginary city by adjusting several environmental factors.
State University of New York
State University of New York: Atomic Absorption and Emission
This module simulates the excitation of hydrogen atoms through irradiation with electromagnetic radiation of different wavelengths.
McGraw Hill
Mc Graw Hill Companies: Origins of the Quantum Theory
An indexing page for Chapter 27 (Origins of the Quantum Theory) of the companion web site for McGraw Hill's Contemporary College Physics textbook. Includes several worthy pages with chapter notes, simple definitions, online computer...
Other
Brockport High School: Energy Levels of Hydrogen Atom
From the Brockport High School Physics Labs web pages. Includes an excellent graphic depicting the energy levels of a hydrogen atoms and portraying the electron level transitions for the Lyman, Balmer, and Paschen series. Includes both...
University of California
Center Science Edu.: Electromagnetic Radiation on Trial
Here is a 1-5 day unit on electromagnetic radiation that features a teacher guide and student activities with extensions.
University of St. Andrews (UK)
University of St. Andrews: Johann Jakob Balmer
Describes the life and scientific contributions of Johann Jakob Balmer. Discussion focuses on Balmer's contributions to the line spectra of hydrogen.