PBS
Science Odyssey: Heisenberg States Uncertainty Principle
Explains the Heisenberg Uncertainty Principle, that it is impossible to know both the momentum and position of an electron, along with describing the impact that it made upon the scientific community upon its introduction in 1927.
Wikimedia
Wikipedia: Werner Heisenberg
In this Spanish-language entry, trace the life and accomplishments of Werner Heisenberg, the great physicist whose work with quantum mechanics resulted in earning the Nobel Prize.
Dartmouth College
Dartmouth College: Chem Lab: Electrons in a Box
This site has an interactive component that allows you to change the mass, number of electrons and the length of the box and see how it affects the energy levels. Requires Java.
Nobel Media AB
The Nobel Prize: Werner Karl Heisenberg Biographical
This website provides information on the life and scientific contributions of Werner Heisenburg, a recipient of the Nobel Prize in Physics for his "creation of quantum mechanics." Read the Prize Presentation Speech in which Professor H....
Other
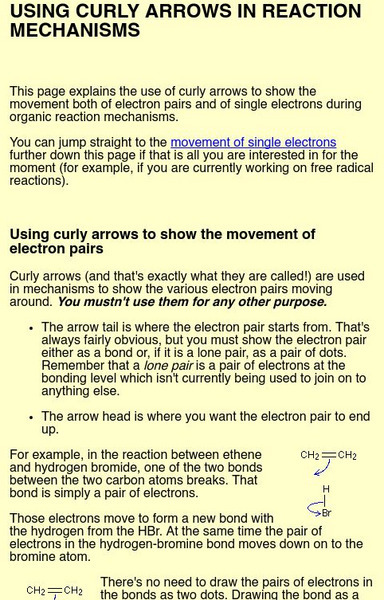
Using Curly Arrows in Reaction Mechanisms
This page explains the use of curly arrows to show the movement both of electron pairs and of single electrons during organic reaction mechanisms.
Curated OER
University of St Andrews: Werner Heisenberg
A detailed biography of Werner Heisenberg. Includes many related links.