Davidson College
Davidson College: Electronic Structure of Coordination Compounds
A list of five virtual chemistry topics with Java applets on the electronic structure of coordination compounds. Covers crystal field theory, CFT energy level splitting, color in gems, the spectrochemical series, and ligand properties.
Davidson College
Davidson College: Visualization of Atomic Orbitals: Hybrid Orbitals
Explains the creation of hybrid orbitals and presents their electron density plots and energy diagrams. Requires Java.
Davidson College
Davidson College: Chemical Kinetics: Integrated Rate Laws
Explains the integrated rate laws for zero-, first-, and second-order reactions. Students then try a virtual stopped-flow experiment to find the rate law and the rate constant for a chemical reaction. Requires Java.
Davidson College
Davidson College: Valence Shell Electron Pair Repulsion (Vsepr) Model
Presents examples of molecules that do not match the expected bond angles in the Valence-Shell Electron-Pair Repulsion Model. Requires Java.
Davidson College
Davidson College: Chemical Equilibria: Calculations With Reaction Stoichiometry
Explains the use of a reaction table when analyzing chemical equilibrium and presents a virtual experiment where the equilibrium constant of a chemical reaction is determined for different scenarios. Requires Java.
Davidson College
Davidson College: Network Solids: Crystalline Solids
Explains the differences between ionic, molecular, metallic, and network crystalline solids. Displays structures for diamond, graphite, fullerene, and silica, accompanied by questions about their chemical bonding. Electron density plots...
Davidson College
Davidson College: Chemical Equilibria
Explains what chemical equilibrium is and the role of the Law of Mass Action. A Java simulation shows the concentration-time curves of a chemical reaction.
Davidson College
Davidson College: Molecular Orbitals of Tetraamminecopper(ii)
A Java applet displays the Ligand Field Theory energy diagram for the Tetraamminecopper(II) ion. When an orbital in the diagram is clicked on, the isosurface is shown.
Davidson College
Davidson College: Sizes of Atomic Orbitals
Using a Java applet, sets of atomic orbitals are compared with different quantum numbers in order to compare the orbital sizes.
Davidson College
Davidson College: Visualization of Atomic Orbitals: P Orbitals
Discusses the geometry of the p orbitals and presents exercises for exploring their shape and orientations. Requires Java.
Davidson College
Davidson College: Visualization of Atomic Orbitals
Discusses the geometry of the d orbitals and presents exercises for exploring their shape and orientations. Requires Java.
Davidson College
Davidson College: Effective Nuclear Charge
Explains what Slater rules are and presents exercises for exploring how they are used to estimate the effective nuclear charge and how well they work compared to the Bohr model expression for orbital sizes. Requires Java.
Davidson College
Davidson College: Effective Nuclear Charge
Explains what the effective nuclear charge is and how electrons can create a shield between a nucleus and an outer electron. Presents exercises for comparing orbital sizes with effective nuclear charges. Requires Java.
Davidson College
Davidson College: Visualization of Atomic Orbitals: S Orbitals
Discusses the geometry of the s orbitals and presents exercises for exploring their shape and orientations. Requires Java.
Davidson College
Davidson College: Phase Changes: Phase Diagram: Part 5: Critical Point
Explains what the critical point in a phase change is and ways that a transition from a liquid to a gas can take place. Students investigate the concepts using a Java simulation.
Davidson College
Davidson College: Valence Shell Electron Pair Repulsion (Vsepr) Model
Presents exercises where students can check their understanding of the Valence-Shell Electron-Pair Repulsion Model by predicting what a molecular shape will be. Requires Java.
Davidson College
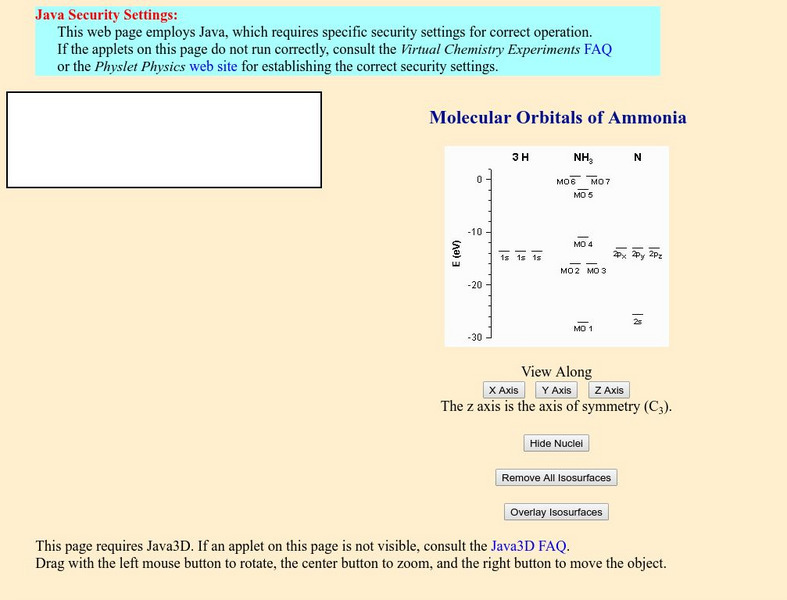
Davidson College: Molecular Orbitals of Ammonia
A Java applet shows a molecular orbital representation of ammonia. When an orbital in the diagram is clicked on, the isosurface is shown. The orbitals for other molecules can be accessed through links at the bottom of the page.
Davidson College
Davidson College: Le Chatelier's Principle: Effect of a Change in Temperature
Explains how Le Chatelier's Principle is used to predict how a temperature change affects a system's chemical equilibrium and presents a virtual experiment where the effect is explored. Requires Java.
Davidson College
Davidson College: Valence Shell Electron Pair Repulsion (Vsepr) Model
Explains the principles of the Valence-Shell Electron-Pair Repulsion Model, and gives examples of the geometry. Requires Java.
Davidson College
Davidson College: Chemical Equilibria: Equilibrium Constant
Explains what the equilibrium constant is and presents a virtual experiment where its value must be determined in two chemical reactions. Requires Java.
Davidson College
Davidson College: Chemical Equilibria: Le Chatelier's Principle
Explains what Le Chatelier's Principle is and presents virtual experiments for exploring its role in chemical equilibrium. Requires Java.
Davidson College
Davidson College: Chemical Equilibria: Uncertainty in Equilibrium Calculations
Discusses and explores the impact of random pressure errors on the equilibrium constant in a chemical reaction. Requires Java.
Davidson College
Davidson College: Le Chatelier's Principle: Effect of a Change in Volume
Explains how Le Chatelier's Principle is used to predict how a volume change affects a system's chemical equilibrium and presents a virtual experiment where the effect is explored. Requires Java.
Davidson College
Davidson College: Molecular Orbitals of Diamminesilver(i)
A Java applet displays the Ligand Field Theory energy diagram for the Diamminesilver(I) ion. When an orbital in the diagram is clicked on, the isosurface is shown.