Curated OER
Electron Arrangement in Atoms
In this electron configuration learning exercise, students fill in 10 blanks with the appropriate terms, they determine if 6 statements are true or false, they match 5 terms with their meanings and they answer two questions about...
Curated OER
Order, Order All Electrons
Students read the periodic table and apply their knowledge of the construction of atoms. They demonstrate reading the electron configuration of an element on the periodic file.
Georgia State University
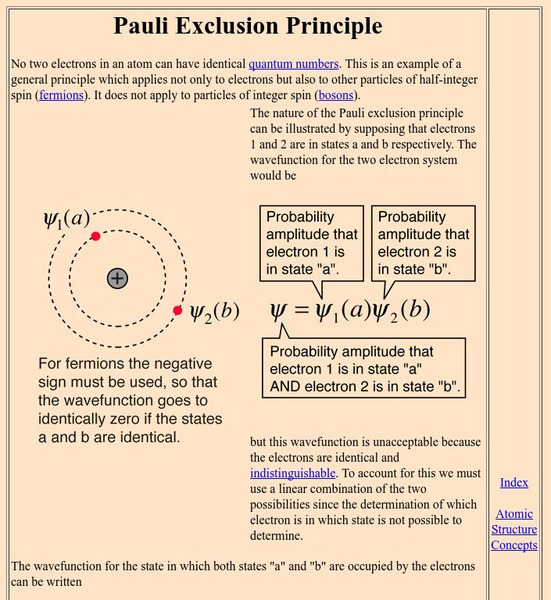
Georgia State University: Hyper Physics: Pauli Exclusion Principle
An explanation of the Pauli Exclusion Principle in mathematical terms.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Electron Clouds and Energy Levels
An explanation of the different types of atomic orbitals, how they are filled according to the Pauli Exclusion Principle, and how many electrons can fit in each electron shell.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Quantum Numbers
Each electron has a set of quantum numbers that specify it's location, orbital, and energy in a unique manner.
CK-12 Foundation
Ck 12: Electron Arrangement in Atoms
[Free Registration/Login may be required to access all resource tools.] In the following online tutorail students will learn how to express the arrangement of electrons in atoms through electron configurations and Lewis valence electron...
CK-12 Foundation
Ck 12: Electron Arrangement in Atoms
[Free Registration/Login may be required to access all resource tools.] The focus in this learning module will be on the complexity of electron arrangement in atoms.
University of St. Andrews (UK)
University of St. Andrews: Wolfgang Pauli
Check out this short look at the life of 1945 Nobel Prize winner, Wolfgang Pauli. Make sure to look at all the links at the bottom of the page too.
Lawrence Berkeley National Laboratory
Berkeley Lab: The Particle Adventure
Visit this site for an interactive tour of the atom and all aspects of particle physics. View the animations available with almost every description on this site. A great place for the fundamentals of particles and forces including a...
Other
University of Winnipeg: The Pauli Exclusion Principle
This site provides information concerning the idea behind the Pauli Exclusion Principle. Includes basic terminology and explanations for understanding.
Wikimedia
Wikipedia: Pauli Exclusion Principle
Wikipedia offers several paragraphs of information on the Pauli exclusion principle that states that no two identical fermions may occupy the same quantum state.
Michigan State University
Michigan State University: Elementary Physics Ii: The Pauli Exclusion Principle
The Pauli Exclusion Principle requires that all electrons in an atom have unique energy levels.
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
Virginia Tech
University of Vermont: Quantum Numbers
This chart highlights the characteristics of the first through fourth electron orbitals in quantum numbers. Also includes information about the Pauli Exclusion Principle.
National High Magnetic Field Laboratory
Magnet Academy: Enrico Fermi
Enrico Fermi was a titan of twentieth-century physics. He outlined the statistical laws that govern the behavior of particles that abide by the Pauli exclusion principle and developed a theoretical model of the atom in his mid-twenties....
National High Magnetic Field Laboratory
Magnet Academy: Wolfgang Pauli
Austrian-born scientist Wolfgang Ernst Pauli made numerous important contributions to twentieth-century theoretical physics, including explaining the Zeeman effect, first postulating the existence of the neutrino, and developing what has...
Khan Academy
Khan Academy: Physical Processes: Electronic Structure: Electron Configurations Article
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. This article describes how this works.
University of Colorado
University of Colorado: Physics 2000: The Pauli Exclusion Principle
This site from the University of Colorado at Boulder has great information on the Pauli Exclusion Principle. Pictures are provided along with links to additional information. The information provided is not in-depth and it is...
Simon Fraser University
Chem1 Virtual Textbook: Electron Spin and the Exclusion Principle
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses electron spin with related topics, such as the Pauli exclusion principle, quantum numbers, and more.
Wikimedia
Wikipedia: Pauli Exclusion Principle
An encyclopedia article from Wikipedia which discusses what the Pauli exclusion principle is, its applications in science, and why its significance.