Curated OER
2007 U.S. National Chemistry Olympiad Local Section Exam
Sixty multiple choice questions cover the entire gamut of chemistry concepts. This is the local section of the U.S. National Chemistry Olympiad, where your chemistry candidates take a shot at entering the national competition. They...
Curated OER
2002 U.S. National Chemistry Olympiad National Exam - Part I
As to be expected from the American Chemical Society Olympiad Examinations Task Force, this 60-question test tops the charts in terms of excellence. It consists entirely of multiple choice questions designed to assess a year's worth of...
Curated OER
Version 001 - Quiz 1
The phase diagram tops this chemistry quiz. Junior scientists answer eight multiple-choice questions as they analyze it and make calculations regarding the heat of vaporization.
Curated OER
Version 001 – Exam 1 – David Laude (53015) 1
A 30-question multiple choice chemistry test challenges takers. Topics touched upon include thermochemistry, equilibrium, behavior of gases, and pH. Problem solving is required in order to answer most of the questions. Other questions...
Curated OER
More Thermochemistry Problems
This two-page assignment covers basic thermochemistry concepts. Chemistry learners identify exothermic and endothermic processes, explain a phase change graph, and draw an energy level diagram. There are no problems to solve, just...
Curated OER
Phases
For this phases worksheet, students rank the chemical compounds by decreasing vapor pressure. Students use a phase diagram to determine if water undergoes any phase transitions during the four steps described. This worksheet has 8...
Curated OER
Chemistry First Marking Period Review
For this chemistry review worksheet, students review terms and concepts covered. Students practice metric conversions, factor labeling, temperature conversions, heat calculations, atomic structure, and gas laws. This worksheet has 3...
Curated OER
Chemical Solutions
In this chemical solutions worksheet, Students determine the significance of the critical point in a phase diagram and rank solutions from weakest to strongest solute-solvent interaction. This worksheet has 4 problems to solve.
Curated OER
Solutions Quiz Review Sheet
In this solutions worksheet, students use a phase diagram to determine the boiling point and molality of the solution. Students determine the electrical conductivity of a saturated solution. This worksheet has nine problems to solve.
Georgia Department of Education
Ga Virtual Learning: Physical Science: Matter and the Atom
Students investigate the structure and parts of the atom, and learn about atomic mass and atomic number. They also explore the differences among solids, liquids, gases, and plasma.
OpenStax
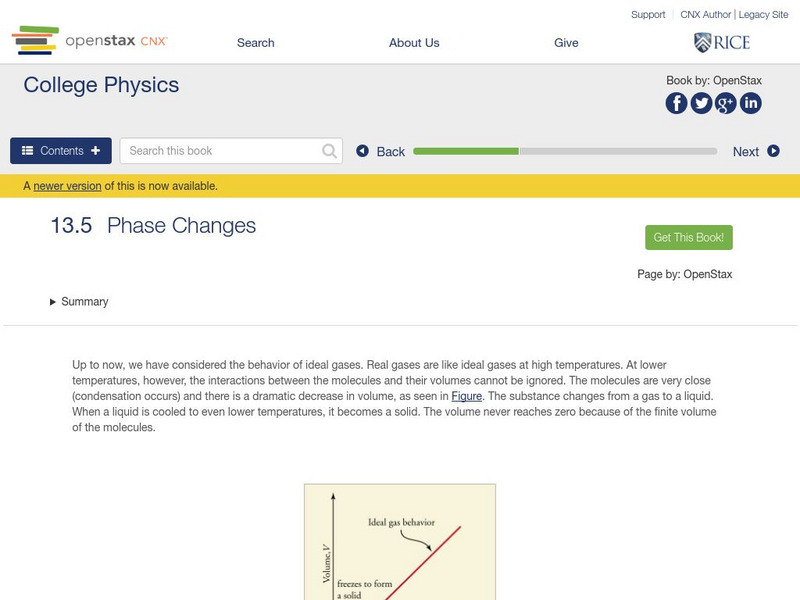
Open Stax: College Physics: Phase Changes
Learn about the phases of matter in this section of a college textbook. Section defines the phases of matter and discusses how to interpret a phase diagram. Also explored is partial pressures and Dalton's Law. Section also includes...
Cosmo Learning
Cosmo Learning: Introduction to Solid State Chemistry
A collection of video lectures from a course that investigates the application of basic chemistry principals to engineering systems. The course draws from industrial practice to give examples of the application in engineering systems. In...
Crescent Public Schools
The Internet Science Room: The Kinetic Theory & Phase Change
This tutorial helps students understand the Kinetic Theory, which explains the effects of temperature and pressure on matter as it goes through phase changes.
University of Sydney (Australia)
University of Sydney: Structure and Properties of Materials/thermal Physics
An exhaustive set of "lecture notes" on various topics in thermal physics (including thermal expansion). Explanations are well done and more interesting than most. Includes both a mathematical and conceptual treatment of topics. Humor,...
MadSci Network
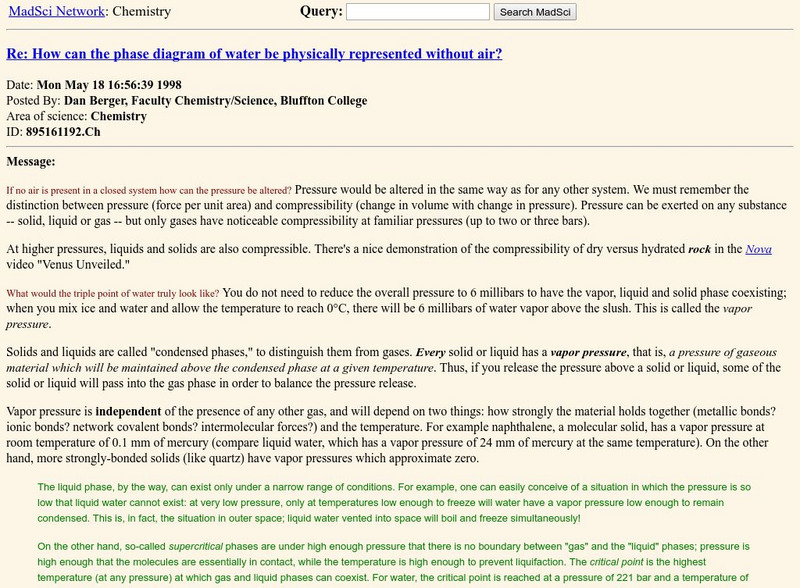
The Mad Scientist Network: Chemistry
The question: "What would the triple point of water truly look like?" is discussed and explained. The phases of matter are described.
California Institute of Technology
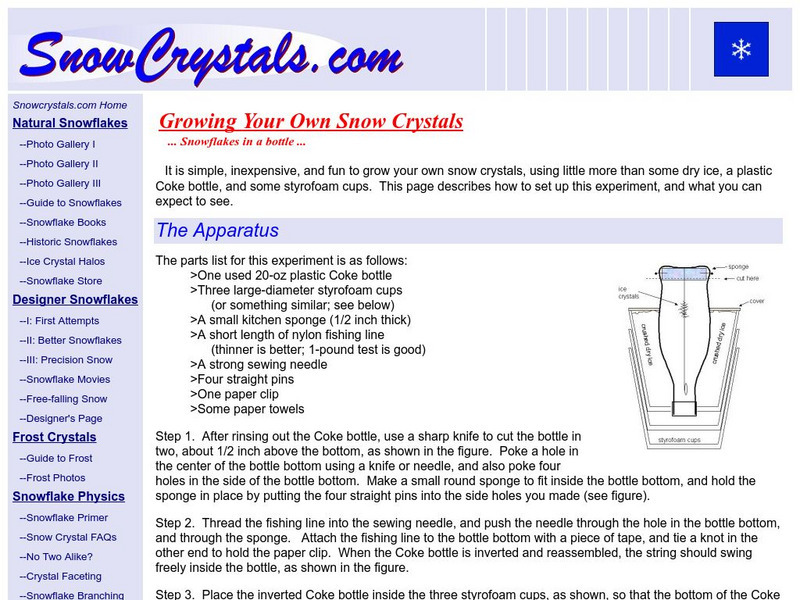
Growing Your Own Snow Crystals
No two snowflakes are the same! Find out if that statement is true by "growing" your own snow flakes and comparing them. You need a coke bottle, dry ice and a Styrofoam cup.