Curated OER
Ionic Formulas
For this compounds worksheet, students write the chemical formula for the chemical ionic formulas given. This worksheet has 25 problems to solve.
Curated OER
Naming Compounds
In this naming compounds worksheet, students are given a chart to determine if the compound they are naming is ionic, covalent or polyatomic. Students practice identifying and naming ionic, covalent and polyatomic compounds. They define...
Curated OER
Naming and Covalent Compounds
In this compounds worksheet, students practice naming compounds and classifying them as ionic, covalent, or polyatomic compounds. This worksheet has 12 fill in the blank and 12 problems to solve.
Frostburg State University
Frostburg State University Chemistry Online: Polyatomic Ions
A good reference page for anyone studying polyatomic ions. Tables included on this page organize polyatomic ions by family and by charge. Information on naming compounds with polyatomic ions is also provided. There is a link to an online...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Oxyanions
Naming polyatomic ions made easy with the help of this site. See how to name them when oxygen is a part of the compound, and learn how some common polyatomic ions received their names.
Science Education Resource Center at Carleton College
Serc: Marshmallow Models
This chemistry lesson will test learners' understanding of VESPR structures. They will use marshmallows and redhots to investigate molecules, acids, and polyatomic ions.
Crescent Public Schools
The Internet Science Room: Chemical Formulas
This illustrated tutorial serves as a foundation for understanding chemical formulas in chemistry.
Other
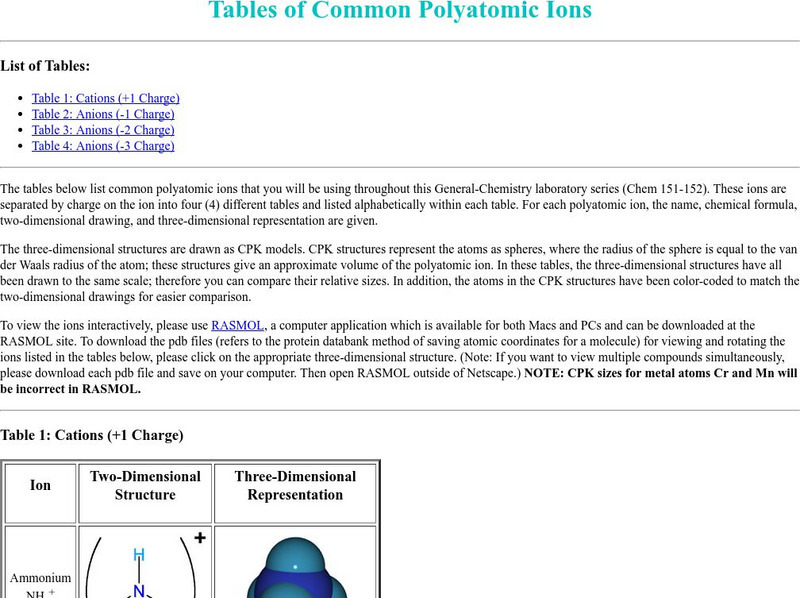
Washington University: Polyatomic Ions
This page from the Department of Chemistry at Washington University provides attractive Lewis dot diagrams and three-dimensional models for many of the common polyatomic ions. The images will make your visit worthwhile.
Quia
Quia: Capitalization of Proper Nouns and Adjectives
A nice online quiz on polyatomic ions. The "joke" answers are a bit distracting, but the page is useful.
Clackamas Community College
Clackamas Community College: Polyatomic Ions
Clackamas Community College provides a great explanation of how polyatomic ions form. This page uses Lewis dot notation to explain polyatomics, and several practice problems are provided.
CK-12 Foundation
Ck 12: Chemistry: Polyatomic Ions
[Free Registration/Login may be required to access all resource tools.] Covers structures of polyatomic ions and polyatomic ion nomenclature.
CK-12 Foundation
Ck 12: Chemistry: Dissociation
[Free Registration/Login may be required to access all resource tools.] Covers dissociation of ions in water, writing equations.
Khan Academy
Khan Academy: Polyatomic Ions
How to name ionic compounds containing common polyatomic ions.
Quia
Quia: Polyatomic Ions
This is a great way to learn your polyatomic ions! Games include; concentration, matching, flash cards, and word search. A link to a reference sheet, on polyatomic ions, is provided.
Sophia Learning
Sophia: Naming Polyatomic Ions: Lesson 2
This lesson will explain that polyatomic ions have assigned names ending in -ate or -ite with a few exceptions, and discuss how ammonium is the only common polyatomic ion. It is 2 of 4 in the series titled "Naming Polyatomic Ions."
Science Struck
Science Struck: A List of Common Polyatomic Ions
Common polyatomic ions are listed in a table. Includes chemical symbol, charge, and oxidation number.