Curated OER
Typical Conceptual Questions for Physics I - Light and Quantum
This is a stellar overview of everything light and quantum! There are 30 multiple choice questions, none of them requiring any mathematical computation. There are a few diagrams to analyze: light rays striking reflective and refractive...
Curated OER
2002 U.S. National Chemistry Olympiad National Exam - Part I
As to be expected from the American Chemical Society Olympiad Examinations Task Force, this 60-question test tops the charts in terms of excellence. It consists entirely of multiple choice questions designed to assess a year's worth of...
Curated OER
Physics 152 Fall 2004 Final Exam, Parts A, B, C, D
At the end of a general physics course focused on light and electricity, you can administer this exam. Concepts covered include electromagnetism, circuits, induction, light rays, lenses and mirrors, characteristics of light, electron...
Curated OER
AP Chemistry Atomic Structure-7 Worksheet
In this atomic structure worksheet, learners solve twelve problems related to wavelength, frequency, electron transitions, emission spectra and quantum number.
Curated OER
Electron Configuration Worksheet
For this chemistry worksheet, students complete the table for each element by writing the electron configuration of the element and giving the values of the four quantum numbers. They also create an energy level diagram for their element...
Georgia State University
Georgia State University: Hyper Physics: Electron Spin
An excellent overview of electron spin, what it means in non-mathematical terms and quantum mechanical terms.
Georgia State University
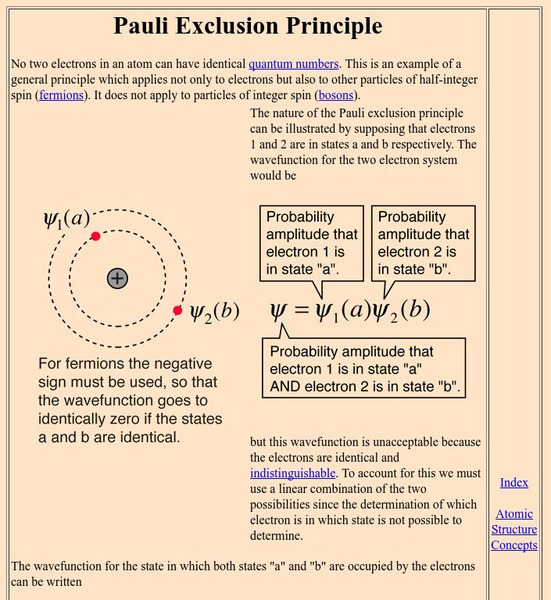
Georgia State University: Hyper Physics: Pauli Exclusion Principle
An explanation of the Pauli Exclusion Principle in mathematical terms.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Electron Clouds and Energy Levels
An explanation of the different types of atomic orbitals, how they are filled according to the Pauli Exclusion Principle, and how many electrons can fit in each electron shell.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Quantum Numbers
Each electron has a set of quantum numbers that specify it's location, orbital, and energy in a unique manner.
Michael Blaber, PhD
Florida State University: Electronic Structure of Atoms: Electron Configurations
Florida State University provides this article on electronic configurations of atoms and Hund's rule.
Vision Learning
Visionlearning: Atomic Theory: Wave Particle Duality and the Electron
An explanation of advanced atomic theory based on developed concepts from earlier scientific experimentation.
Georgia Department of Education
Ga Virtual Learning: Special Relativity and Quantum Mechanics
In this interactive tutorial students explore special relativity and quantum mechanics. Learn basic tenets of the theory of special relativity and the relationship between mass and energy. Also discover the evidence for the particle...
Other
University of Texas: Tabled Discussion
At this University of Texas site, atomic orbital occupancy, quantum numbers, the Aufbau Principle, and periodic trends are described in detail.
Georgia State University
Georgia State University: Hyper Physics: Hund's Rules
A complete overview of Hund's rules including exceptions.
Simon Fraser University
Chem1 Virtual Textbook: Bohr's Model
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses Niels Bohr and his work with the atom. This part of the discussion, focusing on the model itself, brings up...
Simon Fraser University
Chem1 Virtual Textbook: The Quantum Numbers
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses quantum numbers of electrons in atoms including topics such as principal quantum number and orbitals of the...
Purdue University
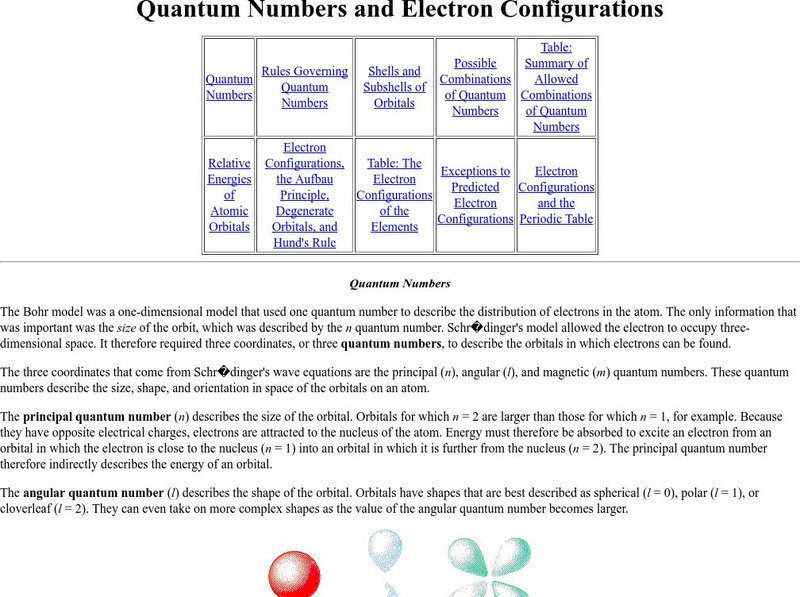
Purdue University: Quantum Numbers & Electron Configurations
This site from the Purdue University provides quantum numbers explained in detail and shown how they specify the atomic orbital of each electron and their energy levels. Electronic configurations are explained. Learning exercises with...
Purdue University
Purdue University: Orbital Shells
This site from the Purdue University provides an overview of atomic orbitals and how quantum numbers specify these orbitals. Includes a model that can be rotated and examined in 3D. Includes learning exercises and answers.
Michael Blaber, PhD
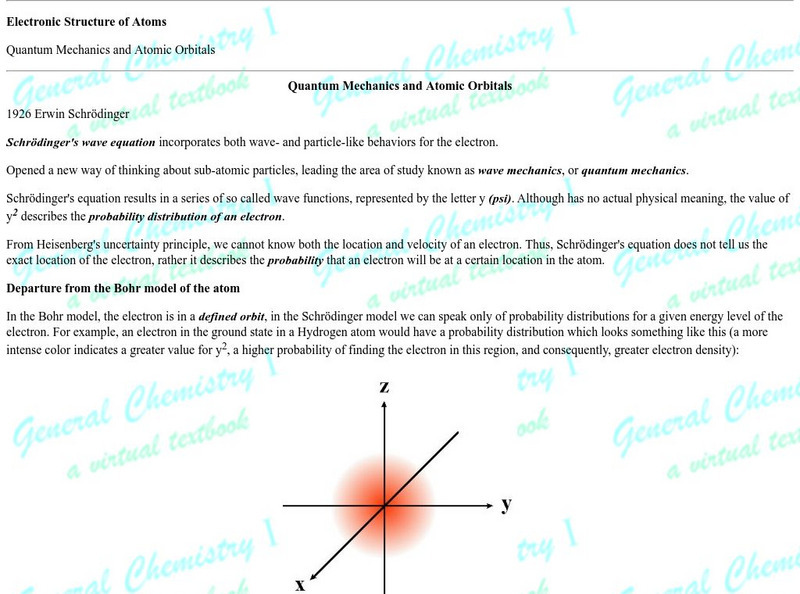
Fsu: Electronic Structure of Atoms: Quantum Mechanics & Atomic Orbitals
This article is provided by Florida State University. Schroedinger wave equation is explained and how quantum numbers are used to describe the energy level of any electron in an atom. The relationship of atomic orbitals to quantum...
Michigan State University
Michigan State University: Elementary Physics Ii: The Pauli Exclusion Principle
The Pauli Exclusion Principle requires that all electrons in an atom have unique energy levels.
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
Frostburg State University
General Chemistry Online: The Periodic Table
This is a teacher's companion guide with lesson plans for the periodic table. It includes learning objectives, lecture notes, links to related sites, answers to frequently asked questions, and a glossary of related terms.
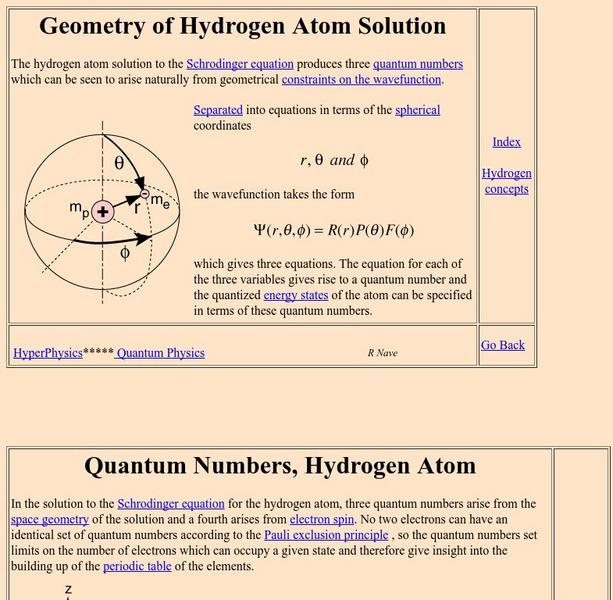
Georgia State University
Georgia State University: Hyper Physics: Quantum Numbers, Hydrogen Atom
This tutorial contains links to explanations of the four different quantum numbers (principal, orbital, magnetic, and spin). Equations for each are provided.