Chemistry Collective
Chem Collective: Camping Problem
Measure the enthalpy of a reaction and then create a solution warm enough to cook food.
Carnegie Mellon University
Chem Collective: Acid Mine Drainage
Students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. In this activity, students examine the chemistry of acidic mine runoff and its effects on river water.
Carnegie Mellon University
Chem Collective: Arsenic in Drinking Water
Set in the context of ground water contamination in Bangladesh, this stoichiometry and analytical chemistry activity examines the issues around identifying wells contaminated with arsenic.
Carnegie Mellon University
Chem Collective: Dna Binding Dyes Scenario
This activity explores the equilibrium of dyes that self-assemble into DNA templates. Students use knowledge of equilibrium and quantitative spectroscopy to explore different dyes that bind selectively to DNA molecules. In this activity,...
Carnegie Mellon University
Chem Collective: Meals Ready to Eat
While camping on the Appalachian Trail, a storm dampens all the fire wood and you must design a chemical reaction to heat your meal. In this activity, students design an experiment in the virtual lab to determine the heat of reaction for...
Carnegie Mellon University
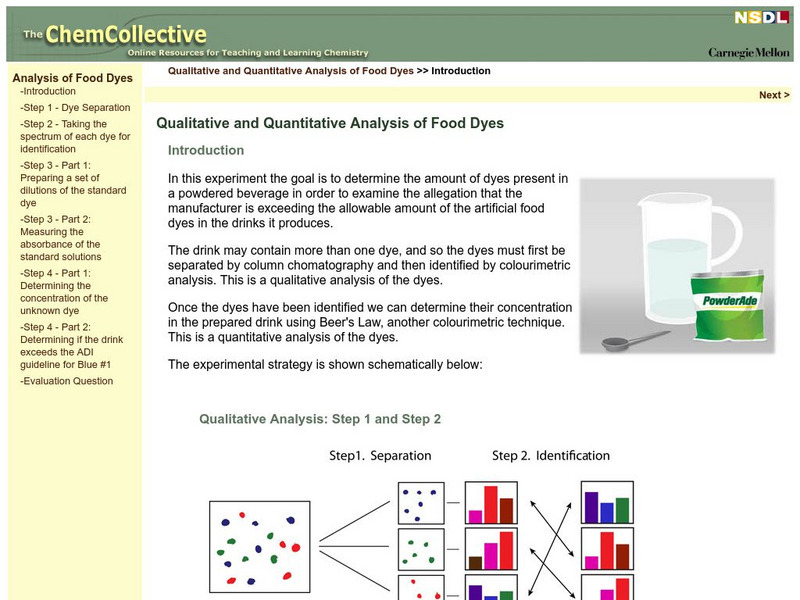
Chem Collective: Qualitative and Quantitative Analysis of Food Dyes
An interactive tutorial to determine the amount of dyes present in a powdered beverage in order to examine the allegation that the manufacturer is exceeding the allowable amount of the artificial food dyes in the drinks it produces.he...
Chemistry Collective
Chem Collective: Determining Reactants and Products in a Solution of Dna
In this limiting reagents problem, students are given specific concentrations of DNA solutions and are asked to predict what products and reactants will remain after a specific volumes are mixed and reaction has occurred.
Chemistry Collective
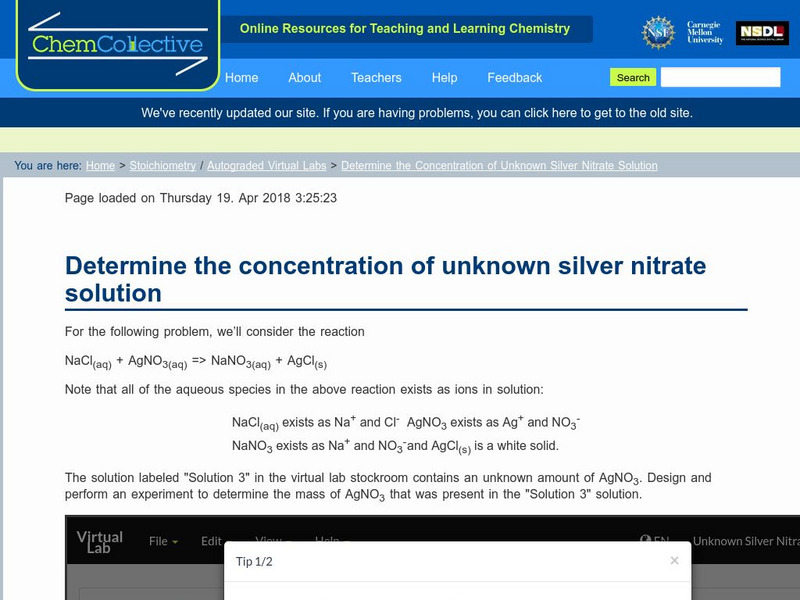
Chem Collective: Determine the Concentration of Unknown Silver Nitrate Solution
In this limiting reagents problem, students are asked to determine the amount of silver nitrate dissolved in a solution by performing a reaction with solid NaCl. In this randomized activity, each student is given a different unknown...
Chemistry Collective
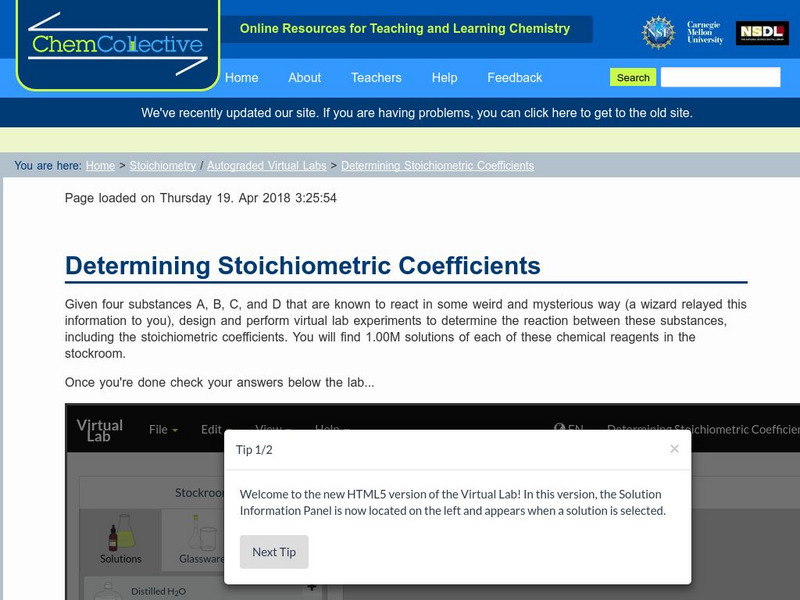
Chem Collective: Determining Stoichiometric Coefficients
Students use the virtual lab to determine how four unknown substances react with each other including their stoichiometric coefficients. In this randomized activity, each student receives a different reaction and students can check their...
Chemistry Collective
Chem Collective: Coffee Problem
In this activity, students use knowledge of specific heat capacity to mix together hot coffee and cold milk to create a solution of coffee at a desired temperature. In this randomized problem, each student is given a different final...
Chemistry Collective
Chem Collective: Unknown Weak Acid Problem
Perform an experiment to determine the pKa and concentration of an unknown acid.
Chemistry Collective
Chem Collective: Unknown Weak Acid Problem Bonus
Perform an experiment to determine the concentrations of a mixture of two acids.
Chemistry Collective
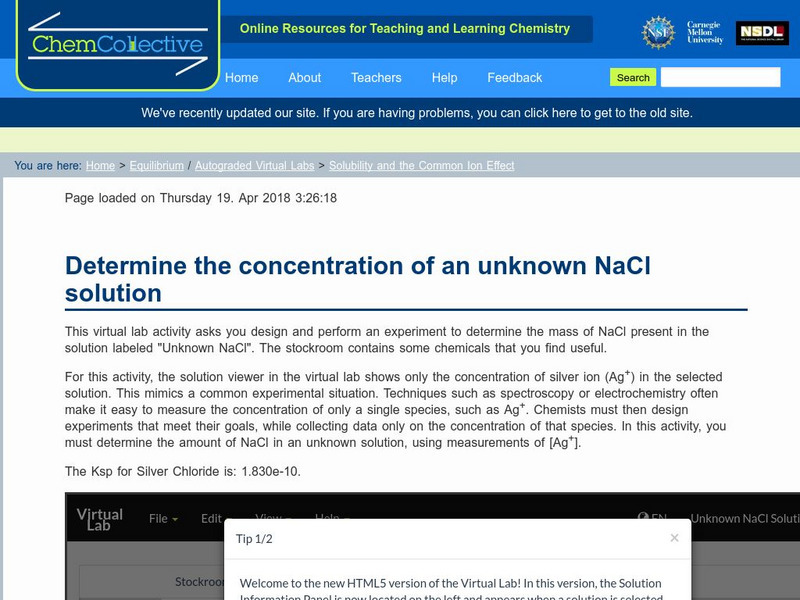
Chem Collective: Solubility and the Common Ion Effect
Determine the concentration of an unknown NaCl solution using the common ion effect.
Chemistry Collective
Chem Collective: Metal Ligand Binding
Use silver chloride to determine the binding constant for a metal ligand complex.
Chemistry Collective
Chem Collective: Determine the Concentration of the Unknown Strong Acid
Perform a titration using an indicator to determine the concentration of an HCl solution.
Chemistry Collective
Chem Collective: Designing a Buffer Solution With a Specific P H
Create a buffer solution at a specific pH using a weak acid and its conjugate base.
Chemistry Collective
Chem Collective: Determining the Concentration of an Unknown Solution
Using the Virtual Laboratory design, students perform an experiment to determine the concentration of the unknown HCl solution to four significant figures.
Chemistry Collective
Chem Collective: Determining Reagent Concentrations
Using the Virtual Laboratory, design an experiment to quickly determine which of the two reagents, HCl or NaOH, is 10 times more concentrated than the other.
Chemistry Collective
Chem Collective: Determine the Amount of Vitamin C in a Peach
Perform a titration experiment to determine the concentration of absorbic acid in one medium peach.
Chemistry Collective
Chem Collective: Unknown Base Problem
Perform an experiment to determine the pKa and concentration of an unknown weak base.
Chemistry Collective
Chem Collective: Glucose Dilution Problem
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together to create the final 0.025M...
Chemistry Collective
Chem Collective: Acid Dilution Problem
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to mix together to create...
Chemistry Collective
Chem Collective: Cola and Sucrose Concentration Problem
In this activity, students use the virtual lab to prepare a sucrose solution for a soda recipe. They next calculate the concentration of their solution in terms of molarity, percent mass and density. Finally, they compare the density of...
Chemistry Collective
Chem Collective: Making Stock Solutions From Solids
In this activity, students use the virtual lab to create stock solutions starting from solid salts. Students must first calculate the correct amount of solid to make the solution. Next, they prepare the solution using the appropriate...