Curated OER
Solubility of Gases in Liquids
Students observe demonstrations to show the solubility of gases in liquids. In this gases lesson, students discover the relationship between temperature and pressure to and how they affect the solubility of gases in liquids. Students...
Science and Mathematics Initiative for Learning Enhancement (SMILE)
Smile: Solubility of Gases in Liquids
Lab activity where students determine the relationship of pressure and temperature on gas solubility.
CK-12 Foundation
Ck 12: Solubility
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will list examples of solutions made from different solute-solvent combinations and explain three factors that affect the...
CK-12 Foundation
Ck 12: Factors Affecting Solubility
[Free Registration/Login may be required to access all resource tools.] Through readings, videos clips, and review questions, study the factors that affect solubility.
Frostburg State University
Why Does the Solubility of Gases Usually Increase as Temperature Goes Down?
This chemistry course article, "Why does the solubility of gases usually increase as temperature goes down?," provides text and related links on the effects of temperature and pressure on the solubility of gases.
Purdue University
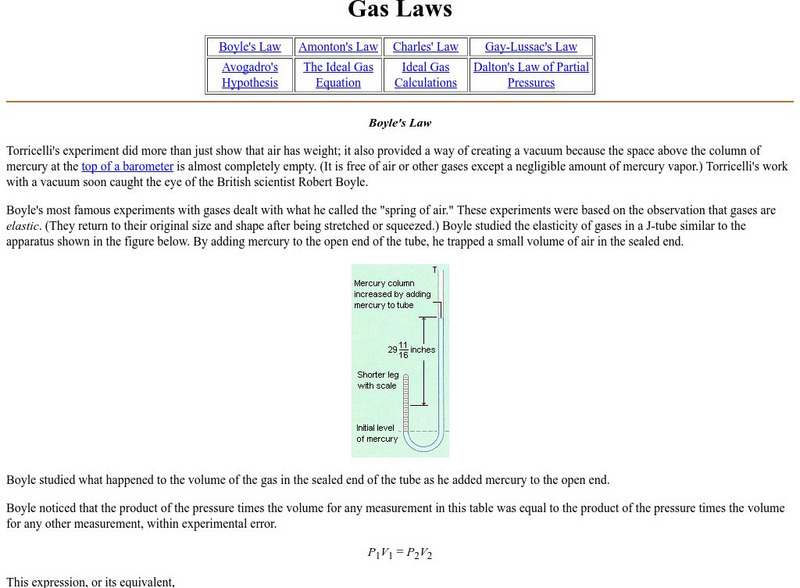
Gas Laws
This chemistry tutorial explains the gas laws (e.g., Boyle's Law) with applications.
University of Nebraska
Microscale: Solubility of Ammonia
This chemistry tutorial lab deals with the solubility of ammonia gas.
CK-12 Foundation
Ck 12: Physical Science: Solubility
[Free Registration/Login may be required to access all resource tools.] Solubility and how temperature and pressure affect it.
Chem Tutor
Chem Tutor: Dissolving Gases Into Liquids
Description of the factors influencing gas solubility. This site also provides other chemistry related questions. Topics include dissolving solids into liquids, concentration, and stoichiometry.
Other
Solubility of Things: Factors Affecting Solubility
Find out how temperature, polarity, pressure, molecular size, and stirring affects the speed of dissolving.