Curated OER
Understanding: Uncertainty
Students discuss the Heisenberg uncertainty principle and how it applies to the subatomic world. Working in groups, they design a model that would help others to understand the uncertainty principle. Written explanations are included...
Other
American Institute of Physics: Werner Heisenberg and the Uncertainty Principle
This resource is an online exhibit covering Heisenberg's physics, career, personal, and political life.
Simon Fraser University
Chem1 Virtual Textbook: Models of Chemical Bonding: Why Do Chemical Bonds Form?
This extensive overview on chemical bonding seeks initially to answer the question, why do chemical bonds form? Information is provided on classical models of chemical bonding and quantum mechanical models of the chemical bond.
Open Curriculum
Open Curriculum: Matter as a Wave
This article helps illustrate the behavior of electrons as waves and the uncertainty principle.
PBS
Science Odyssey: Heisenberg States Uncertainty Principle
Explains the Heisenberg Uncertainty Principle, that it is impossible to know both the momentum and position of an electron, along with describing the impact that it made upon the scientific community upon its introduction in 1927.
Dartmouth College
Dartmouth College: Chem Lab: Electrons in a Box
This site has an interactive component that allows you to change the mass, number of electrons and the length of the box and see how it affects the energy levels. Requires Java.
Famous Scientists
Famous Scientists: Werner Heisenberg
Find out about German scientist, Werner Heisenberg, a theoretical physicist who was one of the key pioneers of quantum mechanics.
Nobel Media AB
The Nobel Prize: Werner Karl Heisenberg Biographical
This website provides information on the life and scientific contributions of Werner Heisenburg, a recipient of the Nobel Prize in Physics for his "creation of quantum mechanics." Read the Prize Presentation Speech in which Professor H....
Science Struck
Science Struck: The Concept of Negative Energy in Physics Simplified
An interesting and detailed discussion of negative energy, how its existence has been proven, and its possible future application in making interstellar space travel possible.
Frostburg State University
General Chemistry Online: Quantum Theory Faq
Find answers to a few common questions when it comes to quantum theory. Explore information about the uncertainty principle as well as radiation and matter.
Simon Fraser University
Chem1 Virtual Textbook: The Uncertainty Principle
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the uncertainty principle and its association with Werner Heisenberg.
Other
Heisenberg Uncertainty Principle
Some references to web links regarding Werner Heisenberg, the uncertainty principle and quantum mechanics.
Other
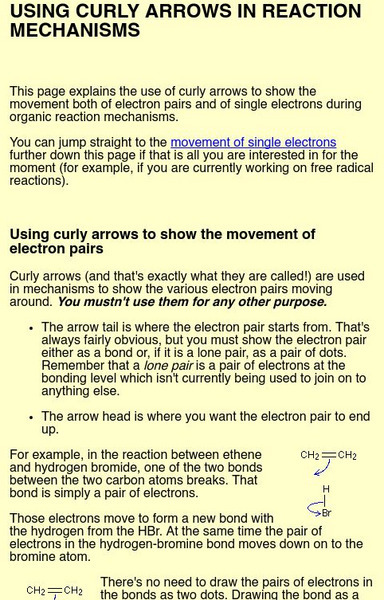
Using Curly Arrows in Reaction Mechanisms
This page explains the use of curly arrows to show the movement both of electron pairs and of single electrons during organic reaction mechanisms.
Physics4kids
Physics4 Kids: Modern Physics: Looking Into Atoms
Get an inside look the subatomic particles of atoms.
Other
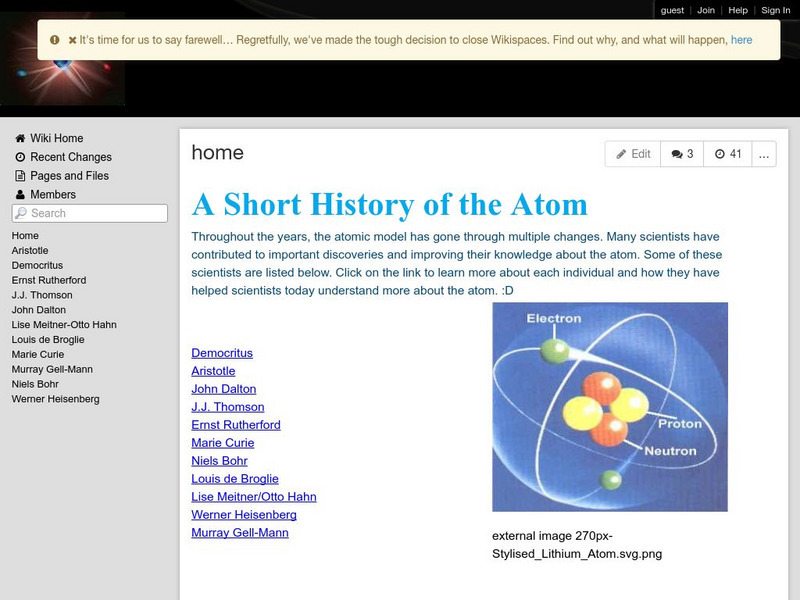
A Short History of the Atom
A classroom wiki where students present profiles of scientists who developed models of the atom or who contributed to the understanding of atomic theory. Covers Democritus, Aristotle, John Dalton, J.J. Thomson, Ernest Rutherford, Marie...
Science Struck
Science Struck: Electron Cloud Theory Explained
Explains the Uncertainty Principle, wave-particle duality, what the electron cloud model is, and how it came about as a result of the Schrodinger equation for Hydrogen.