Curated OER
Practice Final
A full-fledged practice final prepares pupils for their general chemistry final exam. If they complete these 57 multiple-choice questions correctly, they will be well-prepared. Note: even though the questions are multiple-choice, there...
Curated OER
VSEPR THEORY: BALLOONS AND MOLECULES
High schoolers listen to the teacher introduce VSEPR theory and explain molecular geometry. They use balloons to visualize the shape of orbitals. Students create visuals of molecular orbitals with strings and balloons.
Curated OER
Pauli's Magical Water
Students predict the shape of molecules using VSEPR theory. In this chemistry lesson, students differentiate a polar and nonpolar molecule. They discuss why water's polarity is very important.
Simon Fraser University
Chem1 Virtual Textbook: Molecular Geometry
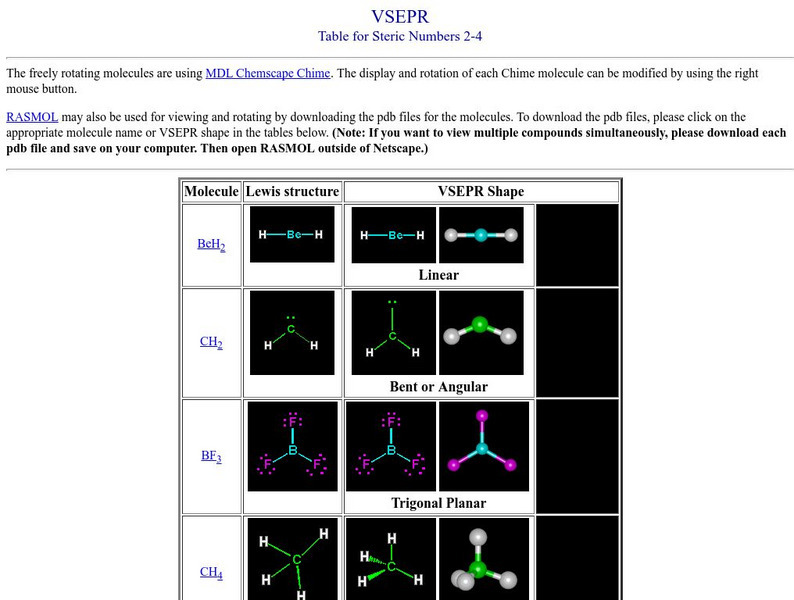
An advanced explanation of the valence shell electron pair repulsion (VSEPR) theory describes specific molecular models involving digonal, trigonal, tetrahedral, and octahedral coordination, as well as central atoms with five bonds....
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Molecule Shapes
Explore molecule shapes by building molecules in 3D. How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare...
CK-12 Foundation
Ck 12: Molecular Geometry
[Free Registration/Login may be required to access all resource tools.] In this interactive learning module, students will learn a technique to predict molecular geometry based on a molecule's Lewis electron dot structure.
Texas Education Agency
Texas Gateway: Valence Shell Electron Pair Repulsion
Why does the shape of a molecule matter? Learn about how the shape of the molecule not only determines the molecule's properties but also whether it reacts or not. This tutorial reviews of VSEPR and molecular geometry.
OpenStax
Open Stax: Andrew R. Barron: Valence Shell Electron Pair Repulsion (Vsepr) Theory
Detailed explanation of Valence Shell Electron Pair Repulsion (VSEPR) Theory with examples and questions for the reader to check comprehension. Also includes step-by-step instructions for correctly building molecules using VSEPR Theory....
Wolfram Research
Wolfram Science World: Valence Shell Electron Pair Repulsion
Good site includes the basics of arrangement, hybridization, and gemetry.
Towson University
Towson University: Shapes of Molecules
This chemistry class printout details the main points of molecular geometry and explains bond hybridization and bond angles.
Other
Us: Valence Shell Electron Pair Repulsion (Vsepr)
An excellent tutorial that examines VSEPR and pairs of valence electrons. The valence shell electron pair repulsion concept is explored using animated models. Includes a VSEPR calculator. Use the toolbar on the left to navigate through...
University of Waterloo (Canada)
University of Waterloo: Vsepr Models
The University of Waterloo provides background in pair repulsion and confidence building questions. Try this one once you think you know what you are doing!
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Chemical Bonding
Take on this self-guided advanced level e-text, and learn about chemical bonding and molecular structure.
McMaster University
Mc Master University: Molecular Structure
This PowerPoint presentation features 33 slides that explain chemical bonding and molecular shape.
Other
Washinton University: Vsepr
This site from the Department of Chemistry at Washington University is a nice site in that it gives you a graphical look at the arrangement of electron pairs. The plug-in CHIME will let you see the 3-D molecules, but it is good even if...