Curated OER
Physics 152 Fall 2004 Final Exam, Parts A, B, C, D
At the end of a general physics course focused on light and electricity, you can administer this exam. Concepts covered include electromagnetism, circuits, induction, light rays, lenses and mirrors, characteristics of light, electron...
Concord Consortium
Concord Consortium: Stem Resources: Atomic Structure
Introduces learners to atomic models of the past and present, focusing on the orbital model and an explanation of its basis. Learners then have the opportunity to "make an atom" and contrast it with an ion, followed by an isotope. The...
Other
Chemistry Is Easy!: How Do You Write Electron Configurations?
With the goal of making chemistry understandable, this site guides the learner through electron configurations with layman's terms, colorful illustrations, and questions to check learning along the way. The "Purney Cheer" is also...
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Exceptions to the Octet Rule
Lists the exceptions to the octet rule and provides a discussion and diagrams explaining each one. Includes clear diagrams illustrating this concept.
Other
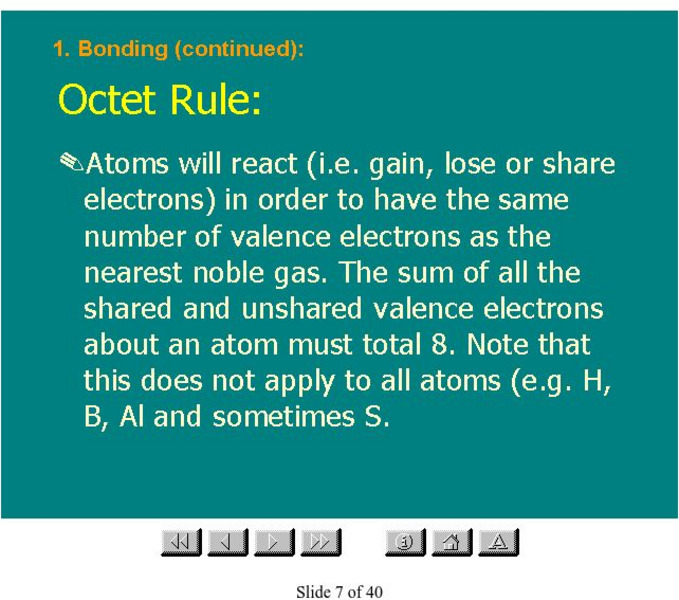
Organic Chemistry: Octet Rule
This slide presentation contains a description of the octet rule, as well as many related topics.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....