American Chemical Society
Middle School Chemistry: Lesson Plans: Forming a Precipitate
Learners investigate the concept of a precipitate by combining two clear, colorless solutions that produce a solid.
American Chemical Society
Middle School Chemistry: Controlling Amount of Products in a Chemical Reaction
Students analyze the chemical equation for the reaction between vinegar and baking soda. They observe that the gas produced in the reaction is also part of the products of the written chemical equation.
PBS
Pbs Learning Media: Acids and Bases: Cabbage Juice Indicator
In this video segment, the ZOOM cast demonstrates how to use cabbage juice to find out if a solution is an acid or a base.
Utah Education Network
Uen: Balancing Act
Students will use wooden atoms to model and balance a chemical reaction.
Concord Consortium
Concord Consortium: Stem Resources: Baggie Chemistry
Observe chemical and physical changes with this lab using everyday household items. Lab includes procedure and online data collection tool where answers can be saved and graded by teacher.
Concord Consortium
Concord Consortium: Stem Resources: Catalysts
Want to see how a catalysts help a reaction happen? In this science simulation, students can experience a reaction that has gone forward due to a catalyst.
Science Museum of Minnesota
Science Museum of Minnesota: Experiment Solving Dissolving
Try this experiment to find out what happens to chalk when vinegar is added to it.
Science Education Resource Center at Carleton College
Serc: Le Chatlier's Principle: Determining Color of Nitrogen Dioxide
Students observe the effects of temperature change on the color of nitrogen dioxide and dinitrogen tetroxide by manipulating glass tubes containing the gases at equilibrium. Then, they write a balanced equation for the reaction and...
Science Education Resource Center at Carleton College
Serc: Hydrogen and Oxygen Gas: An Explosive Interaction
This lab provides the opportunity for students to generate, collect, and test two very common gases, hydrogen and oxygen. They will test the combustion reaction of different proportions of the gases based on the most reactive explosion.
Estrella Mountain Community College
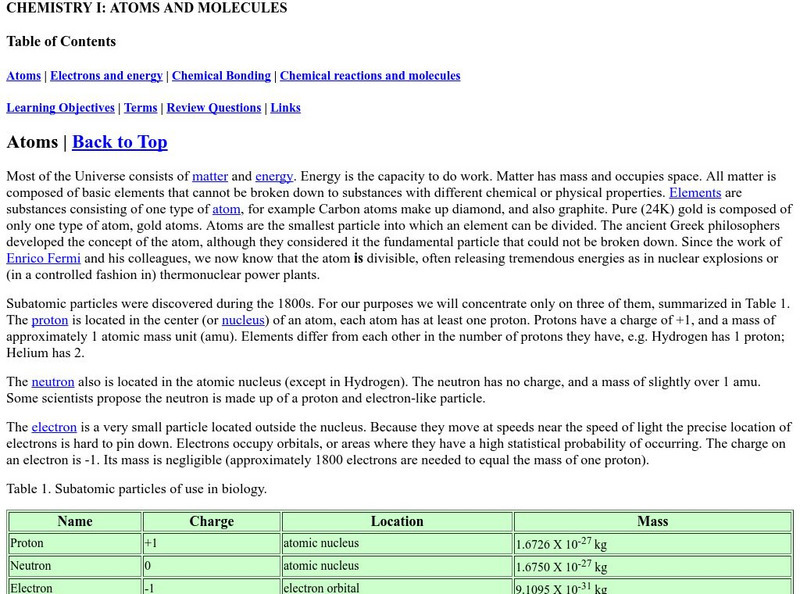
Online Biology Book: Chemistry I: Atoms and Molecules
In this online biology textbook, learn about atoms and molecules as they relate to life. Find out about topics such as electrons and energy, chemical bonding, and chemical reactions.
The Association of the British Pharmaceutical Industry
Abpi: Enzymes
Animated learning exercise about all types of enzymes and their uses. Learn how different types of enzymes work and what effects them.
Crescent Public Schools
The Internet Science Room: Net Ionic Equations
Through example problems and explanation, students learn that net ionic equations attempt to show only the particles involved in a chemical reaction.
Museum of Science
Museum of Science and Industry: Online Science: Create Gas
Follow these simple, step-by-step instructions to create and observe the results of the chemical reaction between vinegar and baking soda.
Other
Widener University: Balancing Chemical Reactions
Practice your balancing chemical equations skills with this interactive exercise. Students are given equations that need to be balanced. Tutorial gives immediate feedback by telling the students they are correct or incorrect. After...
Other
Fun Based Learning: Chemistry: Classic Chembalancer
An exercise for students to practice balancing equations. Exercise gives one equation per page and after the students complete the problem it tells them whether their answer is correct or not. A good basic practice for students who need...
McGraw Hill
Glencoe Biology: Cellular Energy: Chapter Test Practice
Try these fifteen multiple-choice review questions over cellular energy.
Other
Chem Cases: Nutra Sweet: Physical and Chemical Properties
An in-depth look at the physical and chemical properties of aspartame, or NutraSweet.
Center of Science and Industry
Cosi Columbus: Sidewalk Chalk
Make your own sidewalk chalk, and learn about chemical reactions. During the procedure, feel the heat as energy is released in an exothermic reaction.
Sophia Learning
Sophia: Endothermic/exothermic Reactions: Lesson 4
This lesson explains the difference between endothermic and exothermic chemical reactions. It is 4 of 4 in the series titled "Endothermic/Exothermic Reactions."
Sophia Learning
Sophia: Endothermic/exothermic Reactions: Lesson 1
This lesson explains the difference between endothermic and exothermic chemical reactions. It is 1 of 4 in the series titled "Endothermic/Exothermic Reactions."
Chem4kids
Chem4 Kids: Chemical vs. Physical Changes
Identify chemical and physical changes and the differences between the two.
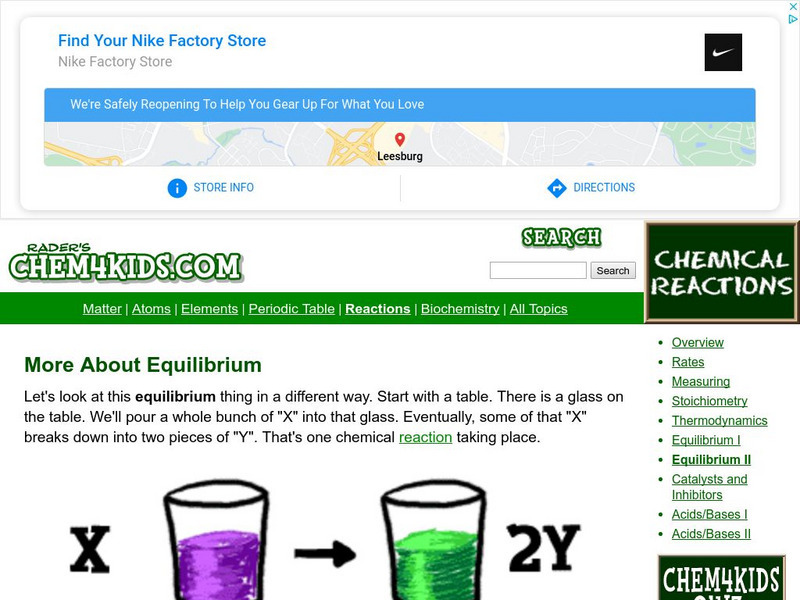
Chem4kids
Chem4 Kids: More About Equilibrium
Equilibrium in chemistry is two reactants combining together and then coming apart to their two parts. Review this resource featuring a way to approach chemical equilibrium.
Chem4kids
Chem4 Kids: Reactions: Names to Know
Acids and bases are scaled. These facts are good knowledge to have when classifying solutions as acidic, basic, or neutral.
CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] Based on the balanced chemical equation, students will calculate the masses or moles of reactants or products generated in a given reaction. They will also convert...