Curated OER
2002 U.S. National Chemistry Olympiad National Exam - Part I
As to be expected from the American Chemical Society Olympiad Examinations Task Force, this 60-question test tops the charts in terms of excellence. It consists entirely of multiple choice questions designed to assess a year's worth of...
Curated OER
2009 U.S. National Chemistry Olympiad National Exam - Part I
The 2009 version of the first part of a national chemistry competition is posted for your use with olympiad hopefuls. Test takers deal with 60 multiple choice questions covering an entire year of chemistry curriculum. Use this to...
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Quantum Numbers
Each electron has a set of quantum numbers that specify it's location, orbital, and energy in a unique manner.
Purdue University
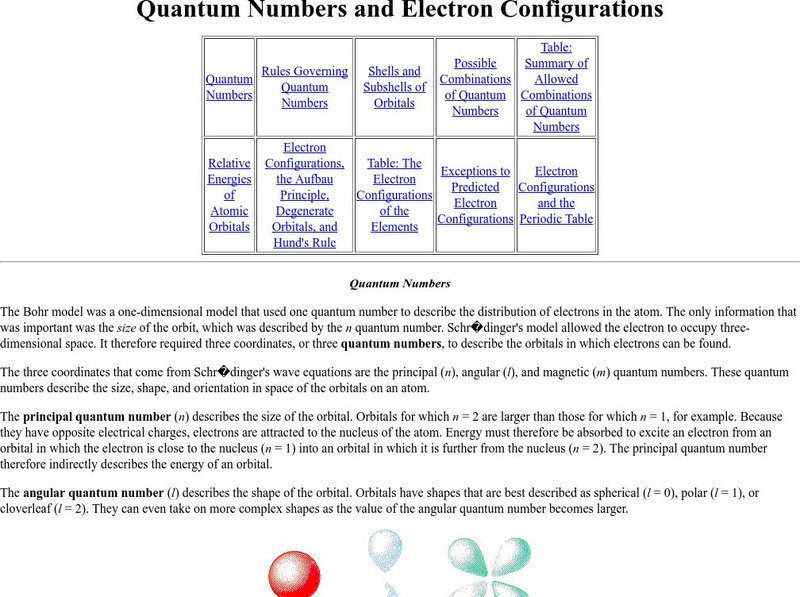
Purdue University: Quantum Numbers & Electron Configurations
This site from the Purdue University provides quantum numbers explained in detail and shown how they specify the atomic orbital of each electron and their energy levels. Electronic configurations are explained. Learning exercises with...
Michael Blaber, PhD
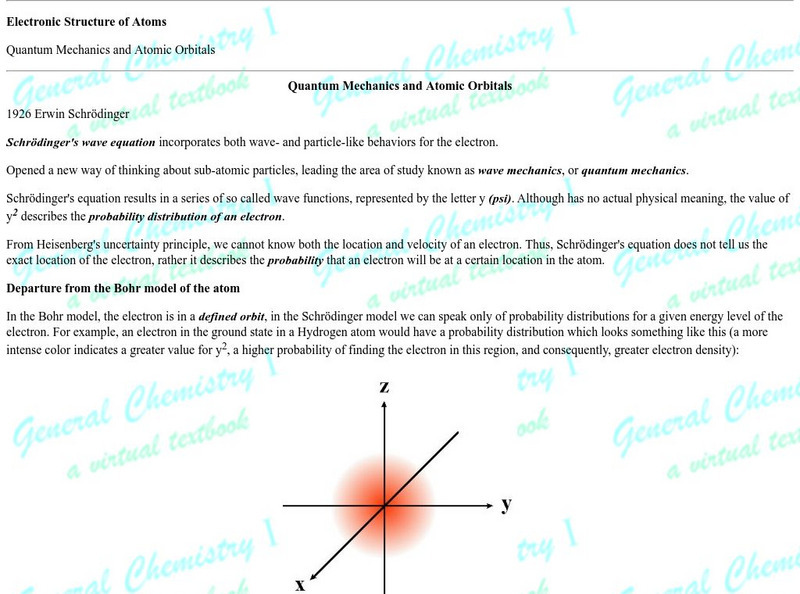
Fsu: Electronic Structure of Atoms: Quantum Mechanics & Atomic Orbitals
This article is provided by Florida State University. Schroedinger wave equation is explained and how quantum numbers are used to describe the energy level of any electron in an atom. The relationship of atomic orbitals to quantum...
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
Georgia State University
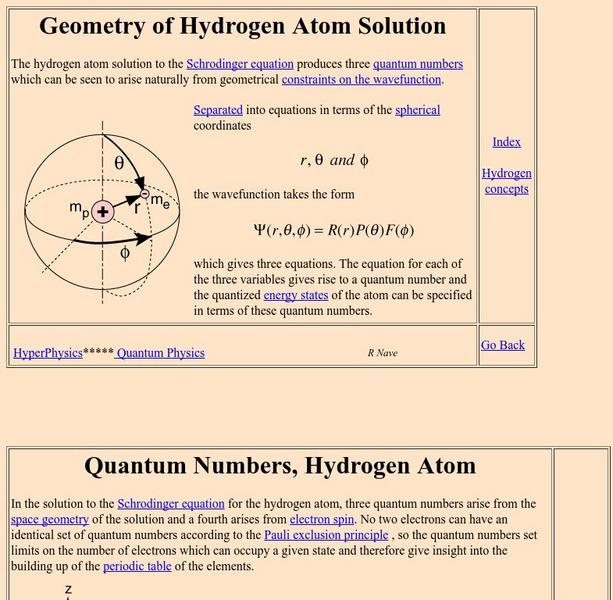
Georgia State University: Hyper Physics: Quantum Numbers, Hydrogen Atom
This tutorial contains links to explanations of the four different quantum numbers (principal, orbital, magnetic, and spin). Equations for each are provided.