Curated OER
Hydrocarbons
For this chemistry worksheet, students use the clues given at the bottom of the sheet to complete the crossword puzzle on hydrocarbons. There are 17 statements to solve and fill in on the puzzle.
Curated OER
"Little" Brothers Share Big Bond
Students listen to the story Little Brothers Share Big Bond and answer comprehension questions. In this lesson on acceptance, students use various areas of reading (vocabulary, fluency, comprehension) to promote compassion and an...
Chemistry Collective
Chem Collective: Metal Ligand Binding
Use silver chloride to determine the binding constant for a metal ligand complex.
Simon Fraser University
Chem1 Virtual Textbook: Models of Chemical Bonding: Why Do Atoms Join Together?
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with chemical bonds and seeks to answer the question, why do chemical bonds form?
Ohio State University
Ohio State University: Electronegativity & Bond Polarity
Excellent graphics help this page explain the relationship between electronegativity and bond polarity.
Michael Blaber, PhD
Florida State University: Basic Concepts of Chemical Bonding: Ionic Bonding
This tutorial explains the basic concepts of ionic bonding, with a good discussion of lattice energy.
Other
University of Massachusetts: Bonds Payable
Issuing bonds is one way a corporation raises capital. This site from the University of Massachusetts Lowell provides a quiz and several problems, complete with solutions, that illustrate the accounting procedures for bonds payable.
Other
Investor Words: What Is a Bond? Definition and Meaning
A definition and description of a bond.
Other
Financial Web: The Independent Financial Portal
This resource presents market news and commentary from financial analysts. It presents quotes, research, and more.
Other
Quote.com
Market updates, news center, quotes, charts, and community features. Good information and links to other sites.
CK-12 Foundation
Ck 12: Plix Series: Resonance: Ozone Resonance
[Free Registration/Login Required] Explore resonance by manipulating the electrons and bonds, and then answer a challenge question about the concept.
Chem4kids
Chem4 Kids: Atoms: Bonding
The website provides a basic explanation of why and how atoms bond together through a covalent bond. Examples with pictures given.
Michael Blaber, PhD
Florida State University: Intermolecular Forces
This article from the Florida State Univeristy provides good information on the various intermolecular forces, including nice diagrams.
Chem Tutor
Chem Tutor: Bonds in General
A summary of bonding terms and the importance of electrons in bonding.
Other
University of Texas at Dallas: Attractions in Compounds
Explanation of attractive forces and energy changes involved in bonding.
CK-12 Foundation
Ck 12: Chemistry: Ionic Bonding
[Free Registration/Login may be required to access all resource tools.] A tutorial about ionic bonding. Learn how and why they form, and the role of energy in their formation.
Biology Pages
Kimball's Biology Pages: Electronegativity
This site, which is a personal site from Kimball's Biology, provides an overview with multiple examples of electronegativity.
University of Massachusetts
University of Massachusetts: Rasmol & Chime
This site from the University of Massachusetts links to downloadable freeware (Rasmol and CHIME) which are required to view 3-D images common on organic chemistry tutorials.
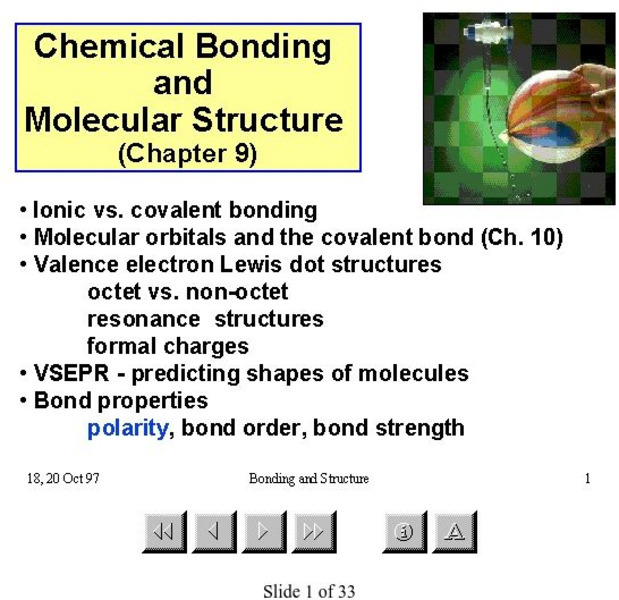
McMaster University
Mc Master University: Molecular Structure
This PowerPoint presentation features 33 slides that explain chemical bonding and molecular shape.
Other
Personal Site: Covalent Bonding Single Bonds
This site is a personal site that gives "a simple view of covalent bonding" including explanations, diagrams, and examples.
Oklahoma State University
Oklahoma State University: Bonding Rules of Thumb
Simple rules to help students identify types of chemical bonding.
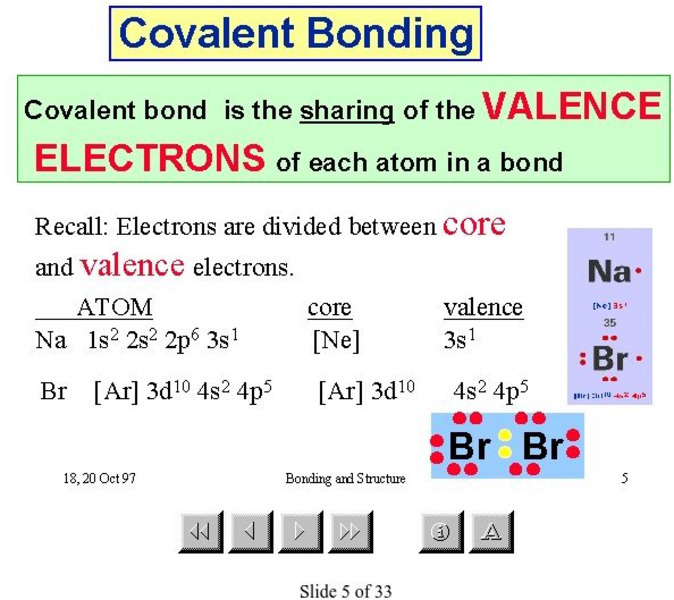
McMaster University
Mc Master University: Covalent Bonding
Slides 5 through 8 in this presentation from the McMaster University explain covalent bonding.