Curated OER
Calorimetry Exercises
In this calorimetry worksheet, students determine the specific heat and the heat of neutralization in the 2 problems they solve.
Curated OER
Combustion
In this combustion pre-lab worksheet, students determine the chemical equation for the reaction, define combustion and exothermic, and describe the molar heat of combustion. This worksheet has 14 short answer questions.
CK-12 Foundation
Ck 12: Heat
[Free Registration/Login may be required to access all resource tools.] Using the law of conservation of energy as a starting off point, students use the specific heat equation to perform calculations that relate mass, specific heat,...
TeachEngineering
Teach Engineering: Counting Calories
The students discover the basics of heat transfer in this activity by constructing a constant pressure calorimeter to determine the heat of solution of potassium chloride in water. They first predict the amount of heat consumed by the...
Chemistry Collective
Chem Collective: Hot/cold Pack Problem: Part 1
Determine the heat of solution for various salts.
Chemistry Collective
Chem Collective: Hot/cold Pack Problem: Part 2
Based on information from Part 1 of the Hot/Cold Pack Problem, design a hot/cold pack.
Science Buddies
Science Buddies: Put Some Energy Into It! Use a Calorimeter to Measure
In this science fair project, use a calorimeter with an attached heating element to measure how water responds to added thermal energy.
Physics Classroom
The Physics Classroom: Thermal Physics
A two-lesson tutorial on thermal energy in the study of physics. Lessons include informational text, interactive activities, animations, and quick, interactive comprehension check-ins.
City University of New York
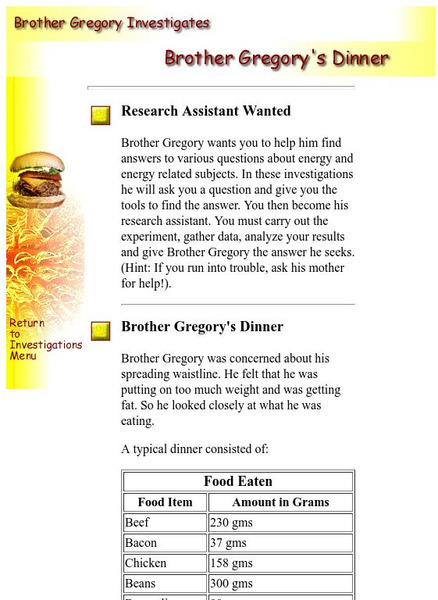
Brother Gregory's Dinner: Food Energy Content
Use a virtual calorimeter to investigate the energy content of various foods. The background information is clear and easy to understand.
Texas Instruments
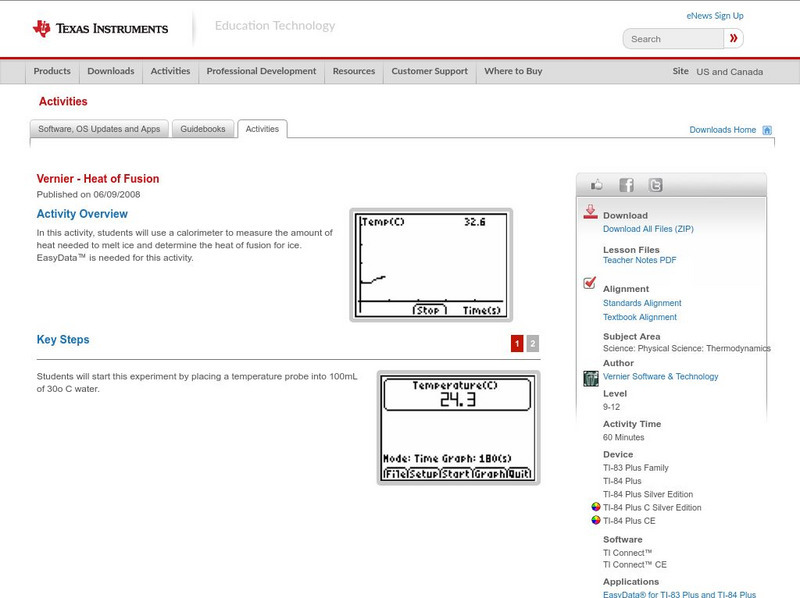
Texas Instruments: Heat of Fusion
In this activity, students can use a calorimeter to measure the amount of heat needed to melt ice and determine the heat of fusion for ice. EasyData is needed for this activity.
CK-12 Foundation
Ck 12: Heat Flow
[Free Registration/Login may be required to access all resource tools.] In this lesson, students study the difference between reactions that absorb versus release heat as well as how to measure this change in energy.
CK-12 Foundation
Ck 12: Chemistry: Calorimetry
[Free Registration/Login may be required to access all resource tools.] Describes the design and function of a calorimeter along with calorimetry sample problems.
University of Sydney (Australia)
University of Sydney: Structure and Properties of Materials/thermal Physics
An exhaustive set of "lecture notes" on various topics in thermal physics (including thermal expansion). Explanations are well done and more interesting than most. Includes both a mathematical and conceptual treatment of topics. Humor,...
Other
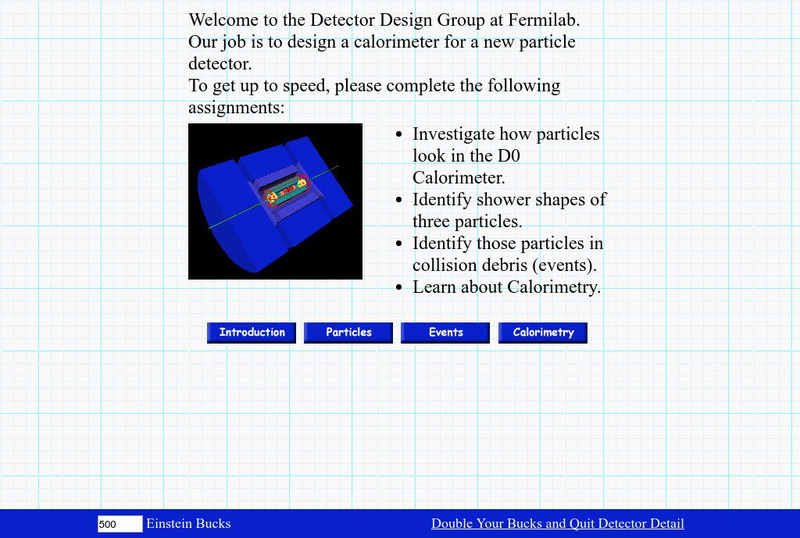
Fermilab: Detector Detail
How do scientists capture and measure the energy in atomic particles? Learn about the design of a calorimeter and how it measures the energy of the atomic particles passing through it.
Physics Classroom
The Physics Classroom: Thermal Physics: Calorimeters and Calorimetry
Students learn about calorimeters and calorimetry in this illustrated, interactive physics tutorial.
Georgia State University
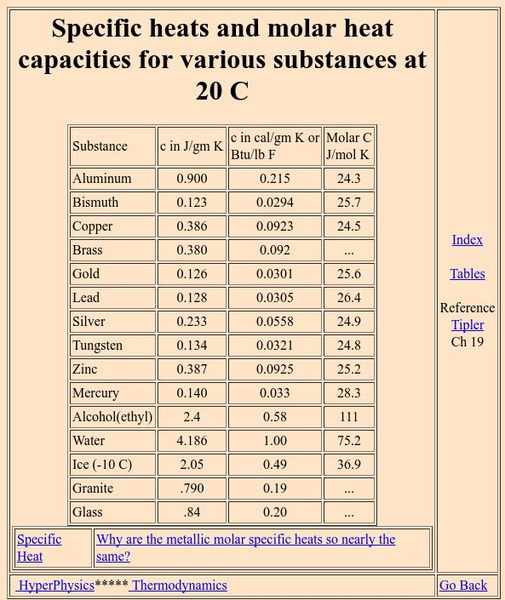
Georgia State University: Hyper Physics: Specific Heats and Molar Heat Capacities
A lengthy listing of values for specific heats and molar heat capacities for a variety of substances at 20 C. An explanation is given for why molar heat capacities for metals are nearly the same.
CK-12 Foundation
Ck 12: Calorimetry
[Free Registration/Login may be required to access all resource tools.] Using diagrams and practice problems, students learn how the units of calories are used to measure the energy of the heat transfer. They also discover how...
PBS
Pbs Teachers: About All You Can Eat: Truth or Consequences
Demonstrate how calories are measured by building a calorimeter to measure the transfer of heat energy during a chemical or physical change. Test and record data on the calories in a peanut.
Other
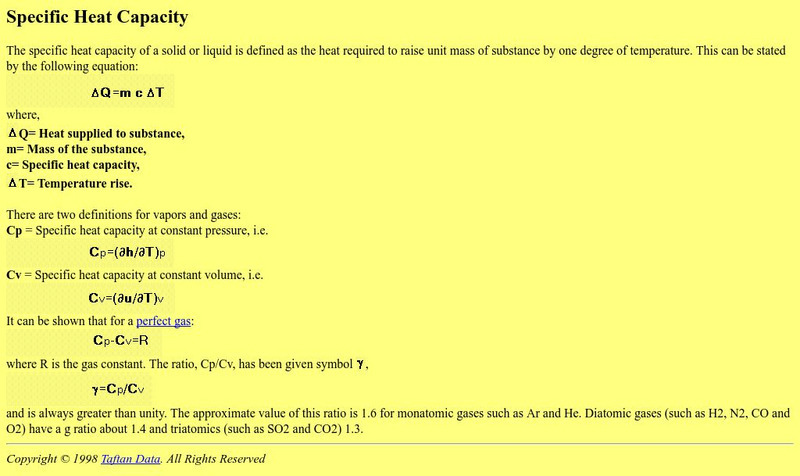
Applied Thermodynamics: Specific Heat Capacity
Specific heat capacity is defined and explained at this site from Applied Thermodynamics. An equation is given. The general equation for the specific heat of an ideal gas is derived and discussed.
University of Nebraska
Do Chem: Simple Heat of Acid Base Reaction
A simple lab activity that allows good data collection on heat of solution.
Other
Physics Labs: Heat of Fusion (Hf) of Ice
A complete set of directions, notes and suggestions for a lab involving the determination of the heat of fusion of ice. Suitable for a student project or lab investigation.
Other
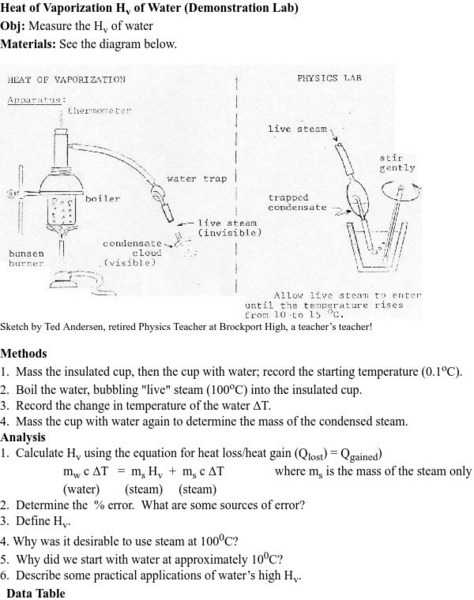
Physics Labs/heat of Vaporization (Hv) of Water
A complete set of directions, notes and suggestions for a demonstration involving the determination of the heat of vaporization of water. Suitable for a student project or lab investigation.
Educaplus (Jesús Peñas Cano)
Educaplus: Calorimetria [In Spanish]
Practice with the calorimeter and calculate the specific heat of different substances.
Other
Physics Labs/specific Heat (C) of Metal
A complete set of directions, notes, and suggestions for a lab involving the determination of the specific heat values for various metals. Suitable for a student project or lab investigation.










![Educaplus: Calorimetria [In Spanish] Activity Educaplus: Calorimetria [In Spanish] Activity](https://content.lessonplanet.com/knovation/original/366822-e58f9ef2399b6d28695cbc1d7c604def.jpg?1661772970)
