Curated OER
I Need Room to Breathe
Seventh graders explore how exercise affects carbon dioxide levels in exhaled air.
Curated OER
SEPARATION OF A STARCH-GLUCOSE MIXTURE USING GEL FILTRATION
Students make a starch-glucose solution and pour it through gel in order to separate the starch from the glucose. They examine how starch is a larger molecule than glucose and test for the presence of these substances using other...
Curated OER
Separating a Starch-Glucose Mixture Using Gel Filtration
Students experiment using the basic principles of gel filtration. They use the gel filtration technique for testing for the presence of specific substances. Students discover that starch is a larger molecule than glucose.
Curated OER
It's a Crash Test, Dummy
Students use the internet to research air bags in cars and trucks. In groups, they discover air bags history and the safety issues surrounding them. They create their own design of a new air bag and test it using a raw egg in...
Curated OER
The Penny Factory
Fourth graders identify the characteristics of a simple physical and chemical change. They describe objects by the properties of the materials from which they are made and separate or sort items using these properties. Students explain...
University of Alberta
The University of Alberta: Separation of Acids, Bases and Neutral Compounds
As a chemist, you will learn to deal with complex mixtures of many compounds. In this interactive activity, you will learn the basis of separations using acid-base extraction. In the virtual lab you will simulate an actual experiment and...
Science Bob Pflugfelder
Science Bob: Lemon Chemistry: An Acid Base Experiement
Science Bob presents instructions for conducting an acid-base experiment using common supplies with information on how it works.
Dartmouth College
Dartmouth College: Acids, Bases, and Buffers 1: Monoprotic and Polyprotic Acids
In this experiment, you will explore the behavior of the monoprotic acid (acetic acid) and the polyprotic acid (phosphoric acid). By titrating, you will examine the acid and conjugate base species present across the pH scale and the...
Dartmouth College
Dartmouth College: Chem Lab: Coordination Chemistry 3.1: Acid/base Analysis
In this experiment, you will examine the acidity of your coordination complex's water ligand. You will determine the acid dissociation constant of the complex by titrating it with a base. There are eight weeks of experiments in this series.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Acid Base Solutions
How do strong and weak acids differ? Use lab tools on your computer to find out! Dip the paper or the probe into solution to measure the pH, or put in the electrodes to measure the conductivity. Then see how concentration and strength...
Science Education Resource Center at Carleton College
Serc: Investigating Acids and Bases: Strong vs Weak
In this chemistry lab activity, students will study acids/bases before learning how titration data is used to determine an unknown concentration. It is an investigation of the pH curve and an assessment of student's understanding of...
Chemistry Collective
Chem Collective: Unknown Weak Acid Problem Bonus
Perform an experiment to determine the concentrations of a mixture of two acids.
Chemistry Collective
Chem Collective: Unknown Base Problem
Perform an experiment to determine the pKa and concentration of an unknown weak base.
Science Education Resource Center at Carleton College
Serc: Acid Base Titration of an Eggshell
In this lab, students are scientists from the Department of Agriculture and presented with the problem that a farmer has brought to them. The farmer believes the problems are due to illegal dumping of PCB's and other chemicals that...
Chemistry Collective
Chem Collective: Standardization of Na Oh With a Khp Solution: Acid Base Titration
Use the Virtual Laboratory to standardize an unknown NaOH solution (approximately 0.2M) to four significant figures via titration with 25.00 mL of a KHP standard solution.
Chemistry Collective
Chem Collective: Determine the Concentration of Acetic Acid in Vinegar
A virtual lab where students determine the concentration of acetic acid in vinegar using a 0.110 M NaOH standard solution and an acid-base indicator, phenolphthalein.
Other
Westminster School: Mixed Indicator Lab
A unique upper level lab showing how to take advantage of the different transition points of two indicators to determine the concentrations of two unknowns at once.
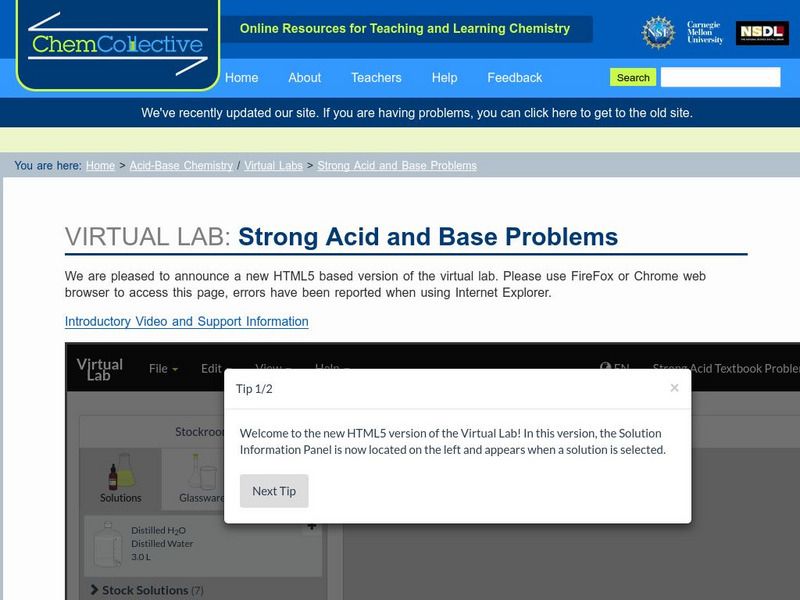
Chemistry Collective
Chem Collective: Strong Acid and Base Problems
Textbook-style strong acid and base problems that can be checked using the Virtual Lab.
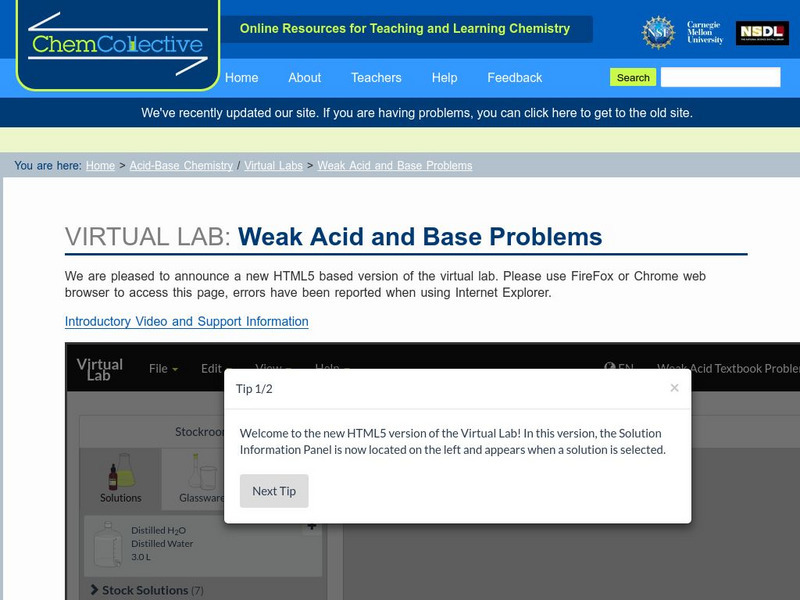
Chemistry Collective
Chem Collective: Weak Acid and Base Problems
Textbook-style weak acid and base problems that can be checked using the Virtual Lab.
Chemistry Collective
Chem Collective: Unknown Weak Acid Problem
Perform an experiment to determine the pKa and concentration of an unknown acid.
American Chemical Society
Inquiry in Action: Color Changes With Acids and Bases
In this lab activity, students will learn how red cabbage can be used as an indicator of an acid or base by looking at its color changes.
Chemistry Collective
Chem Collective: Virtual Lab: Default Virtual Lab Stockroom
Perform virtual lab experiments safely. Pour and mix specific amounts of various solutions on screen and observe changes in temperature and pH. Choose glassware and other tools (ie. bunsen burner, scale) to assist with virtual lab...
Chemistry Collective
Chem Collective: Virtual Lab
Perform virtual lab experiments safely. Pour and mix specific amounts of various solutions on screen and observe changes in temperature and pH. Choose glassware and other tools (ie. bunsen burner, scale) to assist with virtual lab...
Other
Usda: Virtual Labs: The P H Scale & Meter Calibration
In this virtual science lab, students learn how a pH scale works and how to calibrate a pH meter.