Quia
Quia: Common Type Ii Cations
This page links to a number of Java games that are designed to make learning the names, symbols and charges of the various cations fun.
Dartmouth College
Dartmouth College: Chem Lab: Soluble Puzzles, Cations
These interactive chemistry experiments let you choose the chemicals and measurements and provide results that you analyze. Requires Java.
Quia
Quia: Common Monotomic Cations and Anions
This page links to a number of fun Java games, designed to help students learn the names and symbols of various ions. Games include matching, concentration and word search.
Simon Fraser University
Chem1 Virtual Textbook: Naming the Ions
As part of the "Basic Atomics" section of the Virtual Textbook, this site examines the naming system of ions, with specific attention given to cations and anions. Oxyanions and multivalent cations are also discussed.
State University of New York
State University of New York: Hydrolysis
In this module students can learn the pH of a particular salt solution by choosing the anion, the cation and the concentration.
Other
Bsi Education: Qualitative Analysis
The Applied Science resource consists of practical activities that demonstrate the importance of standard procedures in scientific work. Students analyze qualitative data through a variety of activities. Some topics investigated are...
NASA
Nasa: Positive Ions History
As the title suggests, this page from NASA goes over the history of man's study of positive ions. How did we come to know what we now know about cations?
Shodor Education Foundation
Shodor: Background Reading for Ionization Energy
A very complete lesson on ionization energy, including tables and charts to illustrate the ideas presented in the text.
CK-12 Foundation
Ck 12: Ions
[Free Registration/Login may be required to access all resource tools.] In this lesson, students learn ways to predict what type of ion a given element is likely to form.
CK-12 Foundation
Ck 12: Ions
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will be able to determine the number of valence electrons for any element and draw an electron dot diagram for any atom....
Science Education Resource Center at Carleton College
Serc:let's Do Some Bonding! Writing Balanced Formulas and Naming Ionic Compounds
Let's do some bonding! In this activity, students gain practice balancing ionic formulas and naming ionic compounds. They also have the opportunity to meet and 'bond with' students and staff outside the chemistry classroom.
CK-12 Foundation
Ck 12: Chemistry: Polyatomic Ions
[Free Registration/Login may be required to access all resource tools.] Covers structures of polyatomic ions and polyatomic ion nomenclature.
Ohio State University
Ohio State University: Electron Affinity
This page describes electron affinity and the periodic trends of the elements according to their positions on the periodic table.
Science Struck
Science Struck: Examples of Single Replacement Reactions
Explains what a single replacement, or displacement, reaction is; describes the two types - cation and anion replacement reactions; and gives examples of chemical reaction equations for each.
Wikimedia
Wikipedia: Ion
This site is an encyclopedia article from the Wikipedia Encyclopedia on Ion. Throughout the site, links are provided for additional information. The information is somewhat in-depth, but also very factual and interesting.
Other
Aus E Tute: Naming Ionic Compounds
This tutorial offers an explanation and multiple examples of ionic compounds and how to name them appropriately. Includes a small quiz to test your understanding.
Other
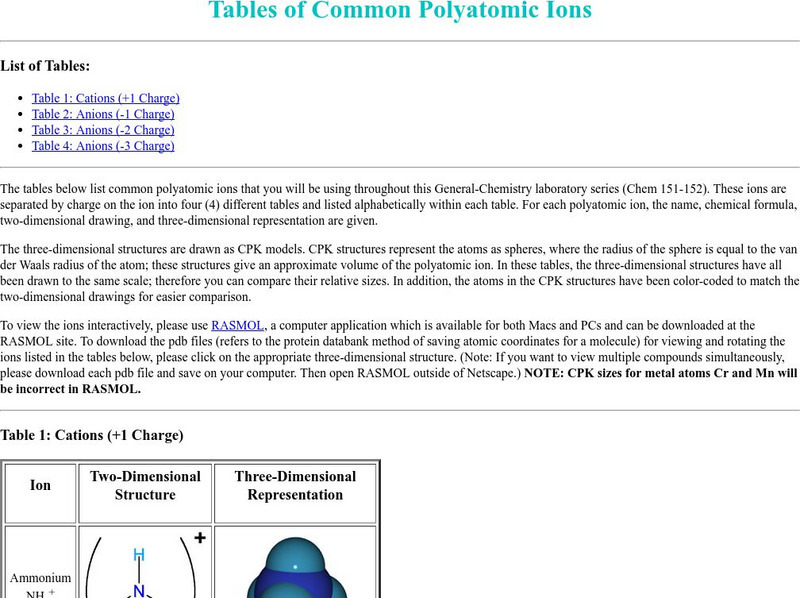
Washington University: Polyatomic Ions
This page from the Department of Chemistry at Washington University provides attractive Lewis dot diagrams and three-dimensional models for many of the common polyatomic ions. The images will make your visit worthwhile.
Science Struck
Science Struck: How to Find Protons, Neutrons and Electrons
Brief explanations of how to determine how many protons, neutrons, and electrons are in an element.
CK-12 Foundation
Ck 12: Periodic Trends
[Free Registration/Login may be required to access all resource tools.] In the following online tutorail students will use the Periodic Table to identify and explain periodic trends, including atomic and ionic radii, electronegativity,...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Predicting Solubility of Ionic Compounds in Water
This easy-to-use chart can help you predict whether ionic compounds will be soluble or insoluble in water. See which cations and anions will dissolve in water and figure out how to determine if unknown compounds are likely to do the same.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Ionic Compounds: Audio Book
Learn how to identify and name ionic compounds in this detailed tutorial. Begin by naming anions and cations and then put them together to form the ionic compounds.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Ionic Nomenclature
Test yourself on ionic nomenclature. Convert either names to formulas or formulas to names. After this quiz, you'll remember how to name cations and anions.
Libre Text
Libre Text: Precipitation Reactions
Precipitation Reactions occur when cations and anions of aqueous solutions combine to form an insoluble ionic solid, called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common...
Frostburg State University
General Chemistry Online: The Periodic Table
This is a teacher's companion guide with lesson plans for the periodic table. It includes learning objectives, lecture notes, links to related sites, answers to frequently asked questions, and a glossary of related terms.