Other
Universe Today; What Is John Dalton's Atomic Model?
Article explaining the legacy of John Dalton. His research into gases led to his gas laws and then his proposed atomic model. The flaws in his atomic theory are discussed also. (Jan. 20, 2016)
Science Struck
Science Struck: Explanation of John Dalton's Atomic Theory
Explains the basic tenets of Dalton's atomic theory.
Other
Iun: Modern Atomic Theory
This is an excellent site with information on the discovery of the atom and the different models. Includes a sample question and answer using Planck's constant.
Upper Canada District School Board
Tom Stretton's Chemistry Pages: Atomic Theory Early Ideas
This online slide show helps students understand the early historical thinking that contributed to modern atomic theory.
Upper Canada District School Board
Tom Stretton's Chemistry Pages: Atomic Theory Modern Ideas
This online slide show helps students understand modern scientific thinking which has shaped modern atomic theory.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Modern Atomic Theory: Study Guide [Pdf]
This study guide provides an overview of the modern atomic theory chapter from "An Introduction to Chemistry. Find chapter goals, map, checklist, web resources and the answer key to the chapter review.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Modern Atomic Theory: Power Point [Pdf]
This power point highlights the main ideas about modern atomic theory from the online textbook "An Introduction to Chemistry". Step by step instructions are given to write electron configurations and draw orbital diagrams. Also includes...
Texas Education Agency
Texas Gateway: Atomic Theory: Dalton, Thomson and Rutherford
This tutorial will help students understand how Dalton, Thomson, and Rutherford contributed to the model of the atom.
Texas Education Agency
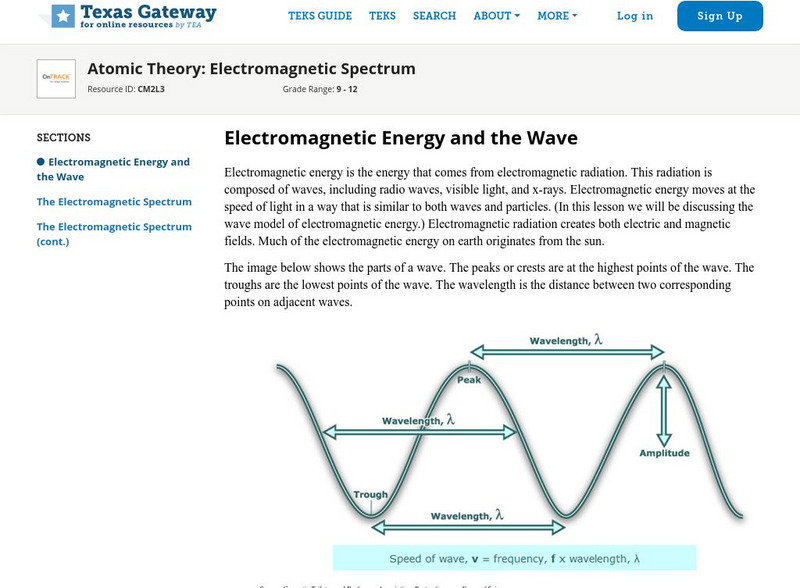
Texas Gateway: Atomic Theory: Electromagnetic Spectrum
This tutorial reviews the electromagnetic spectrum.
Texas Education Agency
Texas Gateway: Matter and Energy: Atomic Structure
This tutorial reviews over the basics of atomic structure.
CK-12 Foundation
Ck 12: Physical Science: Dalton's Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Reintroduction of the idea of the atom and the billiard ball model.
Science Struck
Science Struck: James Chadwick's Atomic Theory and Its Lasting Impact
Explains how James Chadwick came to develop his atomic theory by building upon the work of other scientists.
CK-12 Foundation
Ck 12: Isotopes and Atomic Mass
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will be asked to define atomic number, mass number and understand how isotopes differ from one another. They will be able...
Vision Learning
Visionlearning: Atomic Theory and Structure: The Mole: Its History and Use
An introduction to the mole, a unit of measurement that quantifies atoms and molecules.
Vision Learning
Visionlearning: Atomic Theory and Structure: The Periodic Table
A description of the arrangement of elements on The Periodic Table based on electron configuration.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
Thomas Jefferson National Accelerator Facility
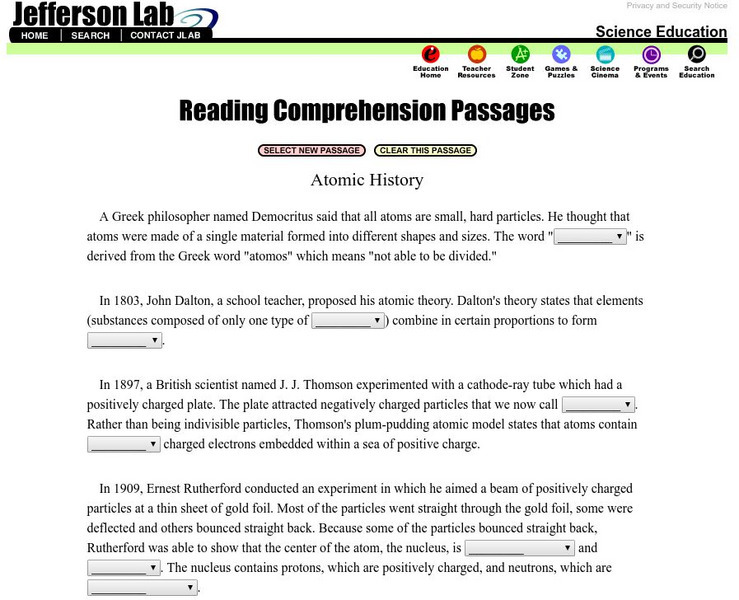
Jefferson Lab: Reading Passages: Atomic History
Read and fill in the blanks of this passage explaining atomic history. Each blank has a dropdown menu with choices. When you finish, click CHECK MY ANSWERS. If you pick a wrong answer, the right answer will be displayed along with your...
OpenStax
Open Stax: Atomic and Molecular Explanation of Pressure and Temperature
A college textbook section that explores the kinetic theory. Learn how the kinetic theory relates to ideal gas law and thermal energy. Section includes problems and questions for the students to answer to ensure understanding. Book can...
Texas Education Agency
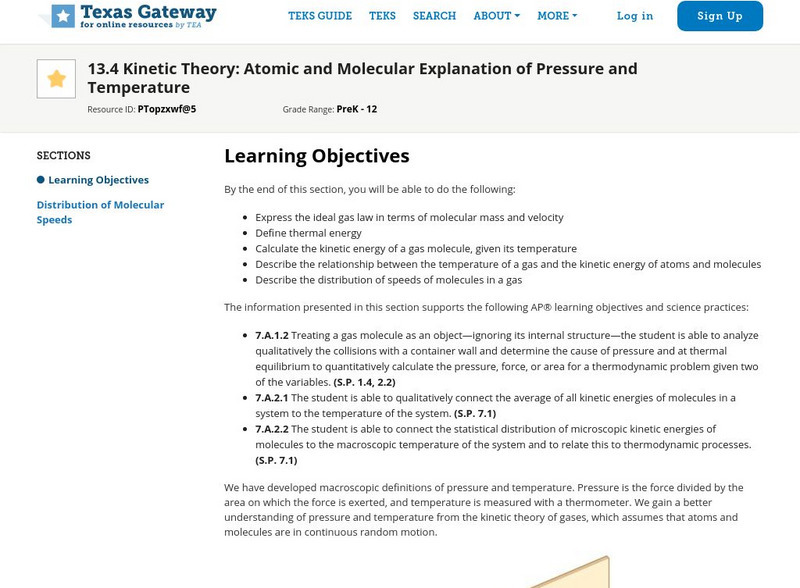
Texas Gateway: Atomic and Molecular Explanation of Pressure and Temperature
We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. Learn more about Kinetic Theory with the following detailed resource...
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
Utah Education Network
Uen: Timeline for the Atom
Learners will examine how significant scientific theories are developed, explore the work of scientists who contributed ideas to the atomic theory, and develop a timeline of key scientists to show how the work of each one built on the...
CK-12 Foundation
Ck 12: The Nuclear Model of the Atom
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will distinguish between the three main subatomic particles and understand the contributions of J. J. Thomson, Robert...
Vision Learning
Visionlearning: Dalton's Playhouse
Travel back in history and visit the laboratories of Priestley, Lavoisier, and others. Take part in simulations of the experiments which laid the foundation for the scientific field of chemistry. Learn about the discoveries which lead to...
Other popular searches
- Modern Atomic Theory
- Modern Day Atomic Theory
- Atomic Theory Time Line
- History of Atomic Theory
- Atomic Theory Worksheet
- Atomic Theory of Matter
- The Atomic Theory
- Atomic Theory Lesson Plans
- John Dalton Atomic Theory
- Progression of Atomic Theory
- Dalton's Atomic Theory
- Atomic Theory Timeline